Data for following 2 compounds have been revised in FactPS :
PbBr4 and Cu(OH)2
The following compound has been added : Li2Nb2O6.
The following compound has been deleted : LiNbO3.
The following 125 compounds have been added to SGPS :
FTfrtz was introduced in FactSage 6.3 (2012).
It is used for the production of nitrate-based fertilizers, from hydrous to anhydrous conditions. It can also be used for calculating the thermodynamic properties and phase equilibria in the fertilizer products, and for some explosives.
The FTfrtz compounds and solutions contain data for 26 pure salts and 14 salt solutions based on the family of ammonium nitrate (NH4NO3), ammonium di-hydrogen phosphate (NH4H2PO4), ammonium chloride (NH4Cl) and ammonium sulfate ((NH4)2SO4). fertilizers with additions of their corresponding potassium salts (and in some cases sodium salts). The model covers the addition of roughly up to 50 wt.% water (H2O).
- minor modifications to the documentation (MAP vs DAP)
- automatic exclusion of N2(g) and several other N-containing gaseous
species (needed for calculations involving NH4NO3)
Al4C5 has been added, and those pure solid metallic compounds (except Al) and solid metallic solutions for which the data are considered to be out-of-date have been removed.
* The carbides (C4- anion) and carbonates (CO32- anion) have been added to the thermodynamic model for the
NaF-AlF3-CaF2-Al2O3 base system.
That is, a thermodynamic model is now available for the
Na+, AlV3+, AlIV3+, Al26+, Ca2+
// F-, O2-, C4-, CO32-, Va- system
{where AlV3+ is the 5-coordinated Al3+, AlIV3+
is the 4-coordinated Al3+, Al26+
represents the dimerized (F-bridged) Al3+, and Va- is an anionic vacancy for metal dissolution}.
The carbides and carbonates were added in order to model the solubility of Al4C3 in presence of dissolved metal
(at Al4C3(s) and Al4O4C(s) saturation) and the solubility of CO2(g), respectively,
in the NaF-AlF3-CaF2-Al2O3 base electrolyte.
At the anode, CO2(g) is evolved and dissolves partially in the bath in the form of carbonates.
The following reactions must be taken into account :
* The following stoichiometric compounds were added to the FThall Compound database :
Na2Ca3Al2F14(s), Na4Ca4Al7F33(s),
Ca12Al14F2O32(s), Na2Mg2Al3F15(s),
Na2CO3(s1, s2, s3, l), CaCO3(s1, s2, l), and Na2Ca(CO3)2(s1, s2).
* Bath: The density of the NaF-AlF3-CaF2-Al2O3-LiF-MgF2 electrolyte
as a function of temperature and composition has been modeled.
In the Menu Window of Equilib, select the FTHall-BathA liquid solution and check the box "include molar volumes".
In the Results Window, the density value (in gram/cm3) calculated from the model is displayed (in parentheses)
at the 2nd line of the block corresponding to the liquid phase.
A system density (in gram/cm3) that takes into account the available density data for all phases at equilibrium
(liquid + one or more solid phases) is displayed below the integral property table.
* Liquid metal: Volumetric properties (density) as a function of temperature were entered for the following pure
liquids in the "Liqu" liquid metal solution of the FThall database :
Al, Ca, Li, Mg and Na. The volumetric properties (density) as a function of temperature and composition were
entered for the Al-Mg binary liquid in the "Liqu" liquid metal solution of the FThall database.
In the Menu Window of Equilib, select the FThall-Liqu liquid solution and check the box "include molar volumes".
* Bath: The viscosity of the NaF-AlF3-CaF2-Al2O3-LiF-MgF2
electrolyte as a function of temperature and composition has been modeled.
In the Menu Window of Equilib, select the FThall-BathA liquid solution and check the box "include molar volumes".
(The viscosity model uses both the molar volume calculated from the density model and the
quadruplet mole fractions calculated from the thermodynamic model, in addition to the viscosity model parameters.)
In the Results Window, the viscosity value (in Pa.s) calculated from the model is displayed at the end of the
block corresponding to the liquid phase.
* Liquid metal: The viscosity of the Al-Mg binary liquid as a function of temperature and composition
has been modeled.
In the Menu Window of Equilib, select the FThall-Liqu liquid metal solution and check the box "include molar volumes".
FTlite was introduced in FactSage 6.1 (2009)
as a major expansion and update of the previous FSlite database for light metal
(mainly Al-based and Mg-based) alloys. The number of fully assessed binary and
ternary systems has more than doubled and a great many previous assessments
have been re-evaluated and re-optimized based upon the most recent data and
improved solution models.
The Mg-Al-Mn-Zn-Li-Ca-Sr-Ce-Y system has been completely re-optimized with the Modified
Quasichemical Model for the liquid phase for short-range ordering (solute-solute interactions).
Note that intermetallic ternary solid solutions are not completely evaluated in some subsystems.
The volumetric properties (density, molar volume) for the Mg-Al-Mn-Zn-(Fe) system (phases
in equilibrium with HCP_A3) are modeled: click the 'use molar volume' option in EQUILIB.
SiC alpha and beta have been changed (drastic change on metastable alpha, added thermal exp. to both).
Name of solid phases changed in Ce-Zn and Y-Zn binary systems.
The database has been updated. The changes are extensive and include the following:
The FTlite light metal alloy database represents a significant update and revision of the previous FTlite alloy database.
It is designed for thermodynamic and phase equilibrium calculations involving Al alloys and Mg alloys.
Calculations involving Ti alloys and Li-Na-K mixtures can also be performed but not to the same extent as for Al- and Mg-alloys.
Al Alloys
Mg Alloys
A total of 622 binary systems (cf. 243 in the previous version of FTlite) have been evaluated,
for most of them over the entire range of composition and for all stable phases.
For around 120 of these binary systems, only the liquid phase mixing parameters are stored.
Several dozen ternary systems have been assessed, and important quaternary systems have also been evaluated.
The database contains 200 solution phases and over 1400 pure compounds
(with more than 1700 stoichiometric phases counting allotropic forms).
All Mg-Zn-RE (RE = rare-earth elements) ternary systems have been evaluated from the Ph.D. thesis of Zhijun Zhu (Polytechnique Montreal).
Estimations of the density at 298.15K for most intermetallic phases (pure compounds) and of end-members of solutions.
Corrections of reported bugs for different solutions (NaZn13, CBCC_A12, etc.).
New documentation file (PDF) for the changes in the solution names between the 6.4, 7.0-7.1 versions.
Ca2Sn(s) has been added to the FTlite compound database.
Corrections have been made to the liquid Sn, FTmisc-Snlq phase.
The FTmisc-Felq liquid iron phase has been updated in FactSage 6.1; it is no longer identical to the liquid iron phase in the FSstel database. This phase is better suited for calculations involving iron and steelmaking processes, whereas the liquid iron phase in the FSstel database is better suited for calculations involving solidification of alloys.
Minor corrections have been made to the database for the Fe-S system
Changes in phase names in FTmisc :
Addition of the (Mn,Fe,Ca,Mg,Cr)S phase with the rocksalt structure for calculations of inclusions in steels in combination with the FToxid-slag phase.
The precise conditions for option 'I' phase selection (sulphide systems) are now stored within the FTmisc database
- the selection of '+' or 'I' will be done for you.
Many new calculated phase diagrams have been added to the documentation for FTmisc.
All compounds and solutions of the former "Light Metals' subsection of FTmisc have been removed.
(These include the solutions FTmisc-LMLQ, FTmisc-FCC, FTmisc-BCC, FTmisc-AlMg and FTmisc-MgSS,
and the stoichiometric compounds Al8Mg5, Al29Mg21, Mg2Si, MgC2, Mg2C3, Al4C3 and SiC.)
These data are out-of-date and have been superseded by the data in the FTlite (and FSlite) database.
FTnucl was introduced in FactSage 7.0 (2015) as a new database developed for the nuclear industry.
FTnucl contains data for pure substances and solutions containing the following elements:
Th, U, Np, Pu, Am
+ Zr, Fe, Ru, Ba + Li, Na, K, Rb, Cs
The FTnucl database can be used for the development of advanced nuclear fuels based on:
It can also be used for estimating the thermodynamic behavior and phase relationships involving fission products (based on Cs. I, Zr, Ru, Ba and Rb, and including noble gases and metallic claddings (Fe, Zr, C).
FTOxCN was introduced in FactSage 6.3 (2012)
as a new high-temperature database of Oxycarbonitride phases
that has been added to the FACT Package of Databases.
Sulfur has been added as a component to the FTOxCN database which can now be used to perform equilibrium
calculations in the Al-(Si-Ca-Mg-Fe)-C-O-N-S system at very high temperatures.
The liquid "Slag" phase is treated as a single solution phase containing all 9 elements,
valid at all temperatures and over all composition ranges of interest.
This phase thus incorporates the high-temperature oxycarbide slag, sulfide-rich liquid and
oxide slags that might appear at lower temperatures, oxynitride liquids, etc.,
all in one solution (with possible immiscibility gaps, of course).
Changes in Phase names:
Changes in phase names in FToxid :
Update to FToxid-slag for liquid FeO-TiO2-Ti2O3 solutions in better agreement with latest experimental data.
Update to FToxid for the CaO-MgO-NiO-SiO2 system including liquid, monoxide, olivine and pyroxene solutions.
The precise conditions for option 'I' phase selection are now stored within the FToxid database
- the selection of '+' or 'I' will be done for you.
Many new calculated phase diagrams have been added to the documentation for FToxid.
Changes have been made in the FToxid database to simplify the selection of phases for
calculations with the Equilib and Phase Diagram modules.
In most cases now, all solutions and all stoichiometric solid phases from FToxid visible
in the menu window can be selected.
Selection of a stoichiometric compound that is simultaneously an end-member
of a solution phase will not adversely affect the calculation.
Normally, the FactPS database should be used in combination with FToxid to select gaseous species ONLY.
Solid and liquid oxide compounds SHOULD NOT be selected from the FactPS database.
I- or J-options are introduced by default only when they are normally needed.
When there is only a small probability that an I-option or J-option is required,
it is NOT introduced by default. (This is done in order to speed up the calculation.)
However, in all solutions in which a miscibility gap could possibly occur,
this fact is mentioned in the description of that solution.
If in doubt, check.
Even though all solutions and stoichiometric compounds from the FToxid database
can now be selected in most cases, this may result in a lengthy calculation for a multicomponent system.
To speed up the calculation, one can select only those solutions that are likely to form and make all other solutions metastable. If the calculated activity of a metastable solution is > 1, this solution should be selected (made stable) and the calculation repeated.
The FToxid-SLAGG phase for C, N and CN dissolved in molten slag has been removed in FactSage 6.4.
For calculations of the solubility of C and N in molten oxides, use the FTOxCN database.
(1) Systems containing Na2O and K2O
The systems Na2O-Al2O3-CaO-SiO2 and K2O-Al2O3-SiO2 have recently been re-evaluated and re-optimized. The binary systems Na2O-X and K2O-X have been evaluated/optimized for X = Al2O3, SiO2 and TiO2, and the liquid solution is assumed ideal for X = CaO, MgO and MnO. This is intended for evaluation of the effect of Na2O and K2O on equilibria between liquid slag and iron/steel. However, only rough estimation can be made for the liquidus in multicomponent Na2O- and K2O-containing systems that are far from the optimized subsystems mentioned above.
Na was added to the melilite solid solution, which was combined with the gehlenite solid solution. This allows evaluation of the solubility of Na and B in melilite.
Several other solid solutions have been added to the database, such as nepheline, carnegeite, combeite, kalsilite, NaAlO2 and KAlO2. See detailed descriptions of these solutions by clicking on “Description of Solutions” in the documentation for FToxid.
(2) Systems containing BaO
The systems BaO-CaO, BaO-SiO2, BaO-CaO-SiO2, BaO-MnO and BaO-CaO-MnO have recently been optimized for evaluation of the effect of BaO on equilibria between liquid slag and iron/steel.
Several Ba-containing solid solutions have been added to the database, such as BaSiO3, Ba3SiO5, CaSiO3-rich pseudo-wollastonite, walstromite [Ca,Ba][Ba,Ca]CaSi3O9 and T-phase Ba2Ca(Ba,Ca)Si2O8. Ba has also been added to monoxide, wollastonite, alpha-prime Ca2SiO4 and alpha Ca2SiO4 solutions. See detailed descriptions of these solutions by clicking on “Description of Solutions” in the documentation for FToxid.
(3) Systems containing P2O5
For the system P2O5-SiO2-Al2O3-CaO-MgO-BaO-FeOx-MnO-Na2O, all binary P2O5-containing subsystems have been recently evaluated and optimized. In addition, the key subsystems CaO-MgO-P2O5, CaO-SiO2-P2O5, CaO-Al2O3-P2O5 and CaO-FeO-Fe2O3-P2O5 have been optimized and the subsystems Na2O-CaO-P2O5, Na2O-MgO-P2O5 and Na2O-SiO2-P2O5 have been approximately evaluated. This can be used for evaluation of the effect of P2O5 on equilibria among liquid slag, iron/steel and gas (solution FeLQ from the FTmisc database should be used for liquid iron/steel).
Liquidus calculations for P2O5-containing systems that substantially deviate from the optimized subsystems mentioned above may be not accurate.
(4) Oxyfluoride system Ca,Mg,Na,Al,Si//O,F
All binary, ternary and higher order subsystems of the (Ca,Na,Al,Si//O,F) and (Ca,Mg,Al,Si//O,F) systems have been optimized and the results are included into an additional slag solution, which is called FToxid-OXFL. The database can accurately calculate phase equilibria up to more than 50% of fluorides. The calculations can be less accurate when both MgO and Na2O are present in high concentrations (> 20% each). The database can be used even for fluoride systems without oxides, but it is less accurate than the FTsalt database. See detailed descriptions of FToxid-SLAG and FToxid-OXFL.
(5) Feldspar
Feldspar solution NaAlSi3O8 – KAlSi3O8 – CaAl2Si2O8 has been added to the database.
(6) Melilite
Thermodynamic properties of melilite in the following subsystems have been optimized: (Ca,Pb)2[Mg,Fe(II),Fe(III),Al,Zn]{Al,Fe(III),Si}2O7, (Ca,Na)2[Al]{Al,Si}2O7 and (Ca)2[Mg,Al,B]{Al,B,Si}2O7.
See detailed description of FToxid-Mel_ by clicking on “Description of Solutions”.
(1) CaO-MgO-NiO-SiO2 system
The CaO-MgO-NiO-SiO2 system has been reoptimized. The changes are
mostly in the Slag, Monoxide, Melilite, Clino-Pyroxene, Proto-Pyroxene
and Olivine solutions
(2) Systems containing BaO
The whole BaO-Al2O3-B2O3-CaO-MgO-SiO2 system has been optimized, including 15
solid solutions and numerous stoichiometric compounds.
(3) Na2O-FeO-Fe2O3-Al2O3-SiO2 system
The Na2O-FeO-Fe2O3-Al2O3-SiO2 system has been optimized.
The following solutions have been updated: Slag, Monoxide, Clino-pyroxene,
Mullite, Carnegieite, Nepheline, low- and high-T Meta-aluminate/ferrite
(NASl and NASh), Feldspar and beta"-alumina.
(4) Systems containing ZrO
ZrO2-containing binaries and ternaries from the
Al2O3-CaO-MgO-SiO2-ZrO2 system have been reoptimized, as well as the
MnO-ZrO2 binary. Slag, Monoxide and ZrO2-based solid solutions (cubic,
tetragonal, monoclinic) have been updated.
(5) Compound database
The FToxid compound database now includes the volumetric and thermal conductivity
parameters for 52 compounds.
(6) Minor changes
There are also a few minor updates/corrections in several solid
solutions and compounds.
Over 50 new FToxid phase diagrams have added to the 'list of stored phase diagrams'.
(1) SrO
SrO has been added to the database. The whole
SrO-BaO-Al2O3-B2O3-CaO-MgO-SiO2 system has been optimized, including 27
solid solutions and numerous stoichiometric compounds.
(2) CaO-MgO-SiO2 system
Bredigite Ca3(Ca,Mg)4Mg(SiO4)4 solid solution has been added to the
CaO-MgO-SiO2 system.
(3) Calculated phase diagrams
Approx. 50 new FToxid phase diagrams have added to the 'list of stored phase diagrams'.
There are now 421 phase diagrams (was 372 in FactSage 7.1)
including 132 (113) binary systems and 289 (259) ternary systems.
New compound data:-
gaseous S, S2, S3, S4, .., S8; metastable Na2SO3 and K2SO3; stable pure solid and liquid NaCl and KCl.
Temperature-dependent volumetric properties (densities) have been added in the FTsalt-SALT liquid solution for the following 50 pure liquids:
LiF, NaF, KF, RbF, CsF, MgF2, CaF2, LaF3, CeF3, LiBr, NaBr, KBr, RbBr, CsBr, MgBr2, LiI, NaI, KI, RbI, CsI, Li2CO3,
Na2CO3, K2CO3, LiNO3, NaNO3, KNO3, RbNO3, CsNO3, LiOH, NaOH, KOH, Li2SO4, Na2SO4, K2SO4, LiCl, NaCl, KCl, RbCl,
CsCl, MgCl2, CaCl2, SrCl2, BaCl2, MnCl2, ZnCl2, PbCl2, AlCl3, LaCl3, CeCl3, CaO.
Volumetric properties (densities) have been modeled in the FTsalt-SALT liquid solution for the following 13 binary liquids:
LiF-NaF, LiF-MgF2, LiF-CaF2, NaF-MgF2, NaF-CaF2, MgF2-CaF2, NaCl-KCl, NaCl-MgCl2, NaCl-CaCl2, KCl-MgCl2, KCl-CaCl2,
MgCl2-CaCl2, CaF2-CaO.
In particular, satisfactory density calculations can be performed for the NaCl-KCl-MgCl2-CaCl2 quaternary liquid.
Note that the box 'include molar volumes' in the Equilib module must be checked in order to perform density calculations.
The FTsalt-SALT liquid solution has been extended with the addition of ZnCl2. NaCl-KCl-MgCl2-CaCl2-AlCl3-ZnCl2 is a newly
approved sub-system of FTsalt-SALT (corresponding to the ISalt-liquid solution). In particular, all five binary subsystems
involving ZnCl2 as well as the NaCl-KCl-ZnCl2 and KCl-CaCl2-ZnCl2 ternary subsystems have been optimized. The following
compounds have been added to the FTsalt pure compound database: ZnCl2(s,l), Na2ZnCl4(s), K2ZnCl4(s), KZn2Cl5(s), K5Zn4Cl13(s).
The following gaseous species have been added to the FACT53 pure compound database: ZnCl2(g), Zn2Cl4(g), NaZnCl3(g), KZnCl3(g).
The precise conditions for option 'I' phase selection are now stored within the FTsalt database
- the selection of '+' or 'I' will be done for you.
The database has been updated with 20 new solution phases and associated compounds
in the nuclear system LiF, PuF3, PuF4, ThF4,
UF3, UF4, (NiF2-CrF2-CrF3-MoF5).
In FactSage 6.3 the phase selection has been simplified by introducing specific modifications in
the FScopp database files.
These modifications are new to FScopp and are intended to simplify automatic species and phase selection.
The selection of certain solution phases now automatically assigns two-phase immiscibility (I-option)
in those cases where this is deemed appropriate.
Furthermore, certain solution phases will not be shown on the menu pages of the
Equilib or Phase Diagram modules, and hence cannot be selected, if components
essential to that phase are missing in the input reactants.
Consequently, with FactSage 6.3, loading a private Phas*.dat or Equi*.dat file,
stored previously using an earlier version of FactSage, and containing a now restricted
phase, may result in the message "The following solution phase could not be located ..." being generated.
In such cases, calculations can still be made as before since the restricted phase is not stable
in any case as shown from previous calculations.
In the new solution database, one or two solution phase names have been changed.
If, upon loading a private Phas*.dat or Equi*.dat file, stored previously using an earlier
version of FactSage, the message "The following solution phase could not be located ..."
appears, and if it is known from previous calculations that the missing phase is actually stable,
then reference to the phase diagram stored in the documentation will permit the new phase
name to be determined.
Species selection with the Equilib and Phase Diagram modules has been made simpler. (Try it and see.)
Some minor updates. Compound MoS2(s2) removed.
Changes in phase names in FSstel :
Amendments and updates have been made to 10 binary sub-systems:
Al-Mn, Al-N, Al-Zn, C-Mn, C-Mo, Co-Si. Cr-N, Cu-Zn, Fe-Nb, Hf-Mo.
The amendments provide improved descriptions and calculations for steels involving the elements listed.
The database has been updated to incorporate new assessed data for the following binary systems:
C-Zr, Hf-Nb, Hf-V, Hf-W, Hf-Zr, Nb-Ta, Ta-V, Ta-Zr, Ti-Zr, V-W, V-Zr, W-Zr
and for the following ternary and quaternary systems:
Al-C-Fe, Al-C-Mn, Al-C-Fe-Mn, C-Hf-Nb, C-Hf-W, C-Nb-Ta, C-Nb-Ti, C-Nb-V, C-Nb-W, C-Nb-Zr, C-Ta-W, C-Ti-W, C-V-W, C-W-Zr.
Parameters for the Cd-Pb system have been revised.
In FactSage 6.3 phase selection has been simplified by introducing specific modifications in
the FSstel database files.
These modifications are new to FSstel and are intended to simplify automatic species and phase selection.
The selection of certain solution phases now automatically assigns two-phase immiscibility (I-option)
in those cases where this is deemed appropriate.
Furthermore, certain solution phases will not be shown on the menu pages of the
Equilib or Phase Diagram modules, and hence cannot be selected, if components
essential to that phase are missing in the input reactants.
Consequently, with FactSage 6.3, loading a private Phas*.dat or Equi*.dat file,
stored previously using an earlier version of FactSage, and containing a now restricted
phase, may result in the message "The following solution phase could not be located ..." being generated.
In such cases, calculations can still be made as before since the restricted phase is not stable
in any case as shown from previous calculations.
In the new solution database, one or two solution phase names have been changed.
If, upon loading a private Phas*.dat or Equi*.dat file, stored previously using an earlier
version of FactSage, the message "The following solution phase could not be located ..."
appears, and if it is known from previous calculations that the missing phase is actually stable,
then reference to the phase diagram stored in the documentation will permit the new phase
name to be determined.
The FSstel database has been modified to simplify the selection of species
when the Equilib or Phase Diagram modules are being used.
Phases that are not possibly relevant to the calculation at hand will not appear in the menu window.
Furthermore, if one now simply selects all the solution phases from FSstel and all the pure solid phases
from FSstel that appear in the menu window, a correct calculation will result in most cases,
with the I- or J-option (for possible immiscibility) automatically selected if possibly required.
The FSstel database has been updated and recently assessed data relating to galvanizing processes associated with the Fe-Al-Mg-Mn-Zn system have been incorporated.
These include revised data for the Fe-Zn system and parameters allowing representation of the likely influence of small concentrations of Mg and Mn on previously calculated ternary phase equilibria.
In addition, the assessed parameters reported by Danielsen and Hald (Calphad 31 (2007) 505) for the Z-phase,
found in new 8-12% Cr martensitic steels, have been converted to FactSage format and incorporated in the present database.
Individual systems for which parameters have been updated or newly included are:
C-Mn, Fe-Si, Mo-Ti, N-Si, Ni-Si
C-Cr-Fe, C-Cr-Mn, C-Cr-Si, C-Cr-Ti, C-Fe-Mn, C-Fe-W, C-Mn-Si, C-Mn-V, C-Mo-Ti, C-Mo-V, C-N-Ti, C-Ni-Si, C-Ni-Ti, C-Ni-W, C-V-W, Cr-Fe-Mn, Cr-Fe-W, Cr-Mn-N, Cr-Mn-Ni, Cr-Mn-Ti, Cr-Mo-N, Cr-N-N, Cr-N-W, Fe-Mn-N, Fe-Mn-Ni, Fe-Ni-Si, Fe-Ni-Ti, Mn-Ni-V, N-Si-Ti
C-Cr-Fe-Mn, C-Cr-Fe-W, C-Cr-Mo-V, Cr-Fe-Mn-N
- Revision of the description of the Fe-Si-C system.
The description of The Fe-Mn-Si-Al-C system for AHSS calculation is improved.
- Revision of the Fe-Ti-C system.
Better description of TiC formation.
- Revision of the Al-Fe-Zn system.
Inconsistency of Al-Fe-Zn system for Zn galvanizing calculation is resolved.
SGTE(2007) database was introduced in FactSage 6.1 (2009)
as a major expansion and update of the previous SGTE(2004) alloy database
with many more fully assessed systems.
As well, many previous assessments have
been re-evaluated and re-optimized based upon recent data.
SGTE(2011) database was introduced in FactSage 6.4 (2013)
as an update of the previous SGTE(2007)
In FactSage 6.4, the SGTE(2011) database has been modified to simplify the selection of species when the
Equilib or Phase Diagram modules are being used.
Phases that are not possibly relevant to the calculation at hand will not appear in the menu window.
Furthermore, if one now simply selects all the solution phases from SGTE(2011) and all the pure solid phases
from SGTE(2011) that appear in the menu window, a correct calculation will result in most cases,
with the I- or J-option (for possible immiscibility) automatically selected if possibly required.
BINS - the SGTE free binary alloy database -
has been updated to be consistent with the new SGTE(2014) database.
The SGTE(2014) database represents a significant update and revision of the previous SGTE(2011) alloy database.
The 78 elements included in the database are:
From among these elements, there are some 577 completely assessed binary alloy systems, of which over 32 are newly assessed systems and many others have been revised or amended on the basis of newly published experimental information. The database also includes about 141 ternary and 15 higher-order systems for which assessed parameters are available for phases of practical relevance. The systems now incorporate approximately 317 different solution phases and 1166 stoichiometric intermetallic compound phases.
This version of the SGTE Solution Database thus represents a significantly upgraded general alloy database. The database is intended to provide a sound basis for calculations relating to the production, heat treatment, constitution, and application of a wide range of alloy types.
BINS - the SGTE free binary alloy database -
has been updated to be consistent with the new SGTE(2017) database.
The SGTE(2017) database represents a significant update and revision of the previous SGTE(2014) alloy database.
The 79 elements included in the database are,
From among these elements, there are some 603 completely assessed binary alloy systems,
of which 15 are newly assessed systems and
28 others have been revised or amended on the basis of newly published experimental information.
The database also includes about 141 ternary and 20 higher-order systems for which assessed
parameters are available for phases of practical relevance.
The systems now incorporate 318 different solution phases and 1227 compound phases
(mainly stoichiometric intermetallics).
Complete details on SGTE(2017) are given in 'Documentation'
in the FactSage Main Menu.
SGnobl was introduced in FactSage 6.1 (2009)
as a major expansion and update of the previous FSnobl database for noble
metal alloys. The number of fully assessed binary and ternary systems has
nearly doubled and many previous assessments have been re-evaluated and
re-optimized based upon recent data.
The number of binary and ternary systems compared with the previous version of the database is 204 (130) and 122 (7) respectively.
While
experimental data from original publications have been used in many cases, the large increase in system content
is due, in large part, to the use of three major sources of information, namely
Handbook of Ternary Alloy Phase Diagrams, eds. P. Villars, A. Prince, H. Okamoto, ASM, 1997.
Ternary Alloys, eds. G. Petzow, G. Effenberg, VCH Verlagsgesellschaft, Weinheim, Vols.1-3, (1988,1990).
The calculation model for enthalpies of formation, due to X.-Q. Chen and R. Podloucky
(CALPHAD 30 (2006) 266-269), which is based on a revised Miedema method in combination with ab initio results.
Use of these resources has enabled the general scarcity of experimental thermodynamic information for many noble
metal systems to be compensated, while retaining a reasonable level of reliability of the resulting assessments.
In addition, the database has been updated and made compatible with assessments for noble metal-containing systems, originating from work carried out within the framework of COST Action 531, Lead-Free Solders.
(Atlas of Phase Diagrams for Lead-Free Soldering compiled by A.T. Dinsdale, A.Watson, A. Kroupa,
J. Vrestal, A. Zemanova, and J. Vizdal, published in 2008 by the European Science Foundation)
This is a new database from SGTE for alloy systems involving the following elements
which are components of lead-containing and lead-free solders: Ag, Au, Bi, Cu, In, Ni,
Pb, Pd, Sb, Sn, Zn.
SpMCBN was introduced in FactSage 7.0 (2015) as a new database that contains assessed thermodynamic parameters for binary and ternary alloys of high-temperature materials containing carbon, nitrogen, boron, and silicon. The alloys include
Me1-Me2-C, Me1-Me2-N, Me1-Me2-B, Me1-Me2-Si, Me-C-N, Me-C-B, Me-C-Si, Me-N-B, Me-N-Si and Me-B-Si systems.
The database is compatible with assessed data for relevant binary, and some ternary systems from the SGTE2011 Solution Database, but the SpMCBN Database incorporates thermodynamic parameters for very many previously un-assessed systems. Calculations of thermodynamic properties and phase diagrams can be carried out for approximately 180 binary, and over 200 ternary systems, for the individual temperatures or temperature ranges covered by the available experimental information.
The general procedure used in obtaining assessed parameters for the solution and compound phases of the large number of previously un-assessed ternary systems in the SpMCBN Database has been to combine the phase boundary information contained in the ASM Handbook of Ternary Alloy Phase Diagrams with available assessed thermodynamic data for the appropriate binary sub-systems. This has allowed a completely compatible set of values to be derived to describe binary and ternary thermodynamic properties and phase equilibria for a particular system.
The elements included in the Spencer Group SpMCBN refractory database are:
B, C, N, Si with
Al, Ca, Co, Cr, Fe, Hf, Mg, Mn, Mo, Nb, Ni, Re, Sc, Ta, Tc, Ti, V, W, Y, Zr
Approx. 180 new SpMCBN phase diagrams have optimized and added to the 'list of stored phase diagrams' bringing the total to 633.
(1) Amended systems
B-Fe B-Si Hf-N
(2) New binary systems
B-Re B-Tc B-Y C-Re C-Sc C-Tc Co-Hf Co-Sc Co-Ti Cr-Hf Cr-Re Cr-Sc Cr-Y
Fe-Hf Hf-Re Hf-Y Mo-Re Mo-Sc Mo-Tc Mo-Y N-Y Nb-Re Nb-Sc Nb-Y Ni-Re Ni-Sc Ni-Tc
Re-Sc Re-Si Re-Tc Re-Ti Re-V Re-Y Re-Zr Sc-Si Sc-Ta Sc-Ti Sc-W Ta-Y
Tc-Ti Tc-W Ti-Y V-Y W-Y
(3) New ternary systems
Al-B-C Al-B-Co Al-B-Cr Al-B-Fe Al-B-Mo Al-B-N Al-B-Nb Al-B-Ni
Al-B-Re Al-B-Ti Al-B-V Al-B-Zr Al-C-Co Al-C-Cr Al-C-Fe Al-C-Hf
Al-C-Mo Al-C-Nb Al-C-Ni Al-C-Sc Al-C-Ta Al-C-Ti Al-C-V Al-C-W
Al-C-Zr Al-Cr-N Al-Hf-N Al-Mo-N Al-N-Nb Al-N-Ni Al-N-Si Al-N-Ta
Al-N-Ti Al-N-V Al-N-W Al-N-Zr
Ag5Te3 AgBrCH5N AgBrH3N AgBrH9N3 AgCClH5N
AgCNO AgCNS AgC2H3O2 AgClH3N AgClH9N3
AgFH4O2 AgFH8O4 AgIO3 AgN3 Ag2O3
Ag2SO3 Ag2SeO3 Ag2SeO4 AlB3H12 AlB3H12
AlCH2NaO5 AlC3H9 AlCl6Fe AlH12N3O15 Al2C6H18
Al2H12O18S3 Al2Sr Al4Sr Al7Sr8 AmCl2
AmCl2 AmCl3 AmCl3 AmO AsO6Sb3
As2O6Sb2 As3O6Sb As4Se3 As4Se3 AuO4Re
B5Mo3 BCH3O BC2H7O2 BC2H7O2 BC3H9
BC3H9 BC3H9O3 BC3H9O3 BC4H14N BC6H15
BC6H15 BCl3H3P B13Mo6 B4S6 BaBr2H2O7
BaBr2H4O2 BaBr2O6 BaCl2H2O BaCl2H4O2 BaCl2H6O11
BaCl2O4 BaF6Si BaH18O10 BaH2I2O7 BaH2N6O
BaH8O12Re2 BaI2O6 BaO3Se BaO4Se BaO4SrTi
BaSe BeSe BeTe BiNa3O4 Bi2Te
C3Nb4 C5Pu6 C7Nb8 C7Pu8 C7V8
CNp CNb C2Th C2U C17U9
C2CaH2O5 Ca24Cu29O56 Ca10F2O24P6 CaCl2H12O6 CaCl2H8O8
CaFeO6Si2 CaH12I2O12 CaH4O5S CaH4O6Se CaH6O9P2
CaI2O6 CaO6P2 Ca12H14O31Si6 Ce4Ru3 Ce7O12
Ce6O11 Cl7Nb3 Cl8Nb3 CoSb Cr6S7
FeO FeSe Fe2Se2 Fe3Si7 FeTe
Mo9O26 NV2 NiSe Ni7Se8 NiTe
O8Pu5 O19W7 O3W O26W9 S2U
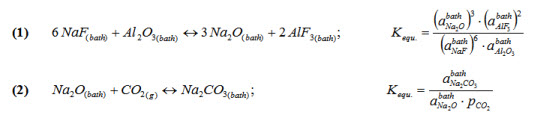
As the activity of Al2O3 is increased in a NaF-AlF3-CaF2 liquid solution,
the activity of Na2O will also increase according to reaction (1).
In the presence of CO2 in the gas at the anode, the activity of Na2CO3 will be
defined by reaction (2) at equilibrium.
Ag, Al, As, Au, B, Ba, Be, Bi, C, Ca, Ce, Co, Cr, Cu, Dy, Er, Eu, Fe,
Ga, Gd, Ge, H, Hf, Hg, Ho, In, K, La, Li, Lu, Mg, Mn, N, Na, Nb, Nd, Ni,
O, P, Pb, Pr, S, Sb, Sc, Si, Sm, Sn, Sr, Ta, Tb, Ti, Tm, V, W, Y, Yb, Zn, Zr
Ag, Al, B, Ba, Be, Bi, C, Ca, Ce, Cr, Cu, Dy, Er, Eu, Fe, Gd, Ge, H, Ho, In, K, La, Li, Lu, Mg, Mn, Na, Nd, Ni, Pb, Pr, Sb, Sc, Si, Sm, Sn, Sr, Tb, Ti, Tm, V, Y, Yb, Zn, Zr
Liq(Matte/Metal) (MAT2): replaced by MAT2A, MAT2B and MAT2C
A major update for the S-Cu-Fe-Ni-Mn-Co-Cr system for calculation of equilibria among liquid and solid sulphide and metal phases. In the earlier versions of FactSage these calculations with Cu and Mn were not possible.
Pyrrhotite (PYRR): replaced by PYRRA, PYRRB and PYRRC
MS2_ solution: name changed to MeS2
M2S_ solution: name changed to M3S2
FCrS_ solution: replaced by the stoichiometric compound FeCr2S4
Fe9S_ solution: replaced by the stoichiometric compound Fe9S10
HCPS – eliminated
+ C, N, O, I
+ He, Ne, Ar, Kr, Xe, Rn
Phase Names in the FToxid solution database in FactSage 5.5 that are no longer
in the FToxid solution database in FactSage 6.1
- All B2O3-containing sub-systems have been completely re-evaluated and re-optimized for all
compositions and all phases
- New B-containing melilite phase (gehlenite) (FToxid-Gehl)
- New compounds Ca2B2SiO7 and Ca11B2Si4O22
- New model for B-containing mullite. Former phases FToxid-MulB and FToxid-MULL
combined into FToxid-Mull
- Update of evaluation of boron in Ca2SiO4 (alpha and alpha-prime)
- Slag/glass phase re-evaluated and re-modeled. The charge compensation effect has been
taken into account.
- Compounds Na3BO3 and NaBSiO4 added
- Completely re-evaluated and re-optimized at all compositions and for all phases
The charge compensation effect has been taken into account for the liquid phase.
- Nepheline, carnegeite and NaAlO2 (FToxid-Neph, FToxid-Carn, FToxid-NASl and
FToxid-NASh) solutions are new, replacing the former stoichiometric compounds
NaAlSiO4 and NaAlO2.
- Evaluation/optimization updated
- Mn-Si-O and Mn-Fe-Si-O systems re-evaluated with the addition of trivalent Mn3+
(Mn2O3).
- New solution phases braunite and bixbyite added (FToxid-Brau and FToxid-Bixb).
- Slag, spinel and olivine phases updated as regards Mn
- FToxid-Rhod (rhodonite) updated to include FeSiO3 and MgSiO3 as components.
Former phase FToxid-MnPy has been merged into FToxid-Rhod
- Mn2O3 now a component of the corundum phase FToxid-Coru
- New tetragonal spinel phase (FToxid-TSPi) added. (Mn3O4 with Fe and Cr in
solution)
- New compounds Mn2O3 and MnO2
- New spinel phase FToxid-SPINB (FeO-CrO-MnO-Cr2O3-Fe2O3-Mn2O3)
- New (Mg,Cr)-olivine phase FToxid-OlivB.
- Systems TiO2-Al2O3-MO (M = Ca, Mg, Mn) re-evaluated and re-optimized
- Ti2O3 added as a component to the corundum FToxid-Coru phase
- Al added as a component to the titanium spinel phase (FToxid-TiSp)
- Former phases FToxid-MeO and FToxid-MONO have been merged into new
FToxid-MeO containing FeO-CaO-MgO-MnO-NiO-CoO + (Al, Fe(III),Cr,Zn in
dilute amounts)
- Completely re-evaluated and re-optimized using all available latest experimental data
and using a new model (Modified Quasichemical Model in the Quadruplet
Approximation) permitting calculations even to high sulphide contents.
Proto-pyroxene (pPyr): replaced by pPyrA, pPyrB and pPyrC
Update to FToxid-slag for more accurate calculation of copper and sulfur solubility in fayalite slags.
Clino-pyroxene (cPyr): replaced by cPyrA and cPyrB
Ortho-pyroxene (oPyr): will appear as a possible solution only if MgO is present
Mg-Zn pyroxene (MgPy): replaced by pPyrC
fcc: replaced by fcc1
The elements Hf and Ta have now been included in the updated version of the database.
This has resulted in the inclusion of assessed data for 7 new binary systems:
C-Hf, Hf-Ta, Hf-Ti, Hf-Ni, Ta-Ti, Si-Ta, Hf-Mo.
bcc: replacec by bcc1
hcp: replaced by hcp1
etc.
Ag, Al, Am, As, Au, B, Ba, Be, Bi, C, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Hf, Hg, Ho, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, N, Na, Nb, Nd, Ni, Np, O, Os, P, Pa, Pb, Pd, Pr, Pt, Pu, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, Zn, Zr
Ag, Al, Am, As, Au, B, Ba, Be, Bi, C, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, H, Hf, Hg, Ho, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, N, Na, Nb, Nd, Ni, Np, O, Os, P, Pa, Pb, Pd, Pr, Pt, Pu, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, Zn, Zr
B-C-Cr B-C-Hf B-C-Mo B-C-Nb B-C-Ni B-C-Sc B-C-Si B-Co-Cr B-Co-Hf
B-Co-Mo B-Co-N B-Co-Nb B-Co-Re B-Co-Si B-Co-Ta B-Co-V B-Co-W
B-Co-Y B-Co-Zr B-Cr-Fe B-Cr-N B-Cr-Sc B-Cr-Y B-Fe-Hf B-Fe-N B-Fe-Sc
B-Hf-Re B-Mo-N B-Mo-Y B-N-Nb B-N-Ni B-N-Ta B-N-Y B-Ni-Re B-Ni-Sc
B-Re-Sc B-Re-Si B-Re-Ta B-Re-Ti B-Re-V B-Re-Y B-Re-Zr B-Sc-W B-Sc-W
B-Si-Y B-W-Y
C-Co-Hf C-Co-Nb C-Co-Si C-Co-Ta C-Co-Ti C-Co-V C-Co-Zr C-Cr-Hf C-Cr-N
C-Cr-N C-Cr-Re C-Fe-Hf C-Hf-N C-Hf-Re C-Mo-Re C-N-W C-Nb-Re C-Re-Si
C-Re-Ta C-Re-Zr C-Sc-Ti C-Sc-Zr C-Si-Ta
Co-Mo-Si Co-N-Nb Co-N-Si Co-N-V Co-Nb-Si Co-Sc-Si Co-Si-Ti Co-Si-W
Cr-Fe-Si Cr-Hf-N Cr-N-Ti Cr-Sc-Si Cr-Si-Y
Fe-Mn-Si Fe-Mo-N Fe-N-Nb Fe-N-Ti Fe-N-W
Hf-N-Si
Mo-N-Ni Mo-N-V Mo-Si-Y
N-Nb-Si N-Nb-Ta N-Ta-Ti N-Si-Ta N-Si-Ti N-Si-Y N-Si-Zr
Nb-Si-V
Ni-Re-Si
Re-Sc-Si Re-Si-Y
Sc-Si-Ta Sc-Si-V Sc-Si-W
Screenshot showing the summary of public databases in FactSage 7.2.