The FactSage FScopp copper alloy database
Summary
TO OBTAIN :
- A LIST OF all the unary, binary AND ternary SYSTEMS WHICH HAVE BEEN ASSESSED
- A LIST OF ALL ASSESSED phases IN EACH OF THE SYSTEMS
- A CALCULATED PHASE DIAGRAM FOR EACH OF THE LISTED BINARY SYSTEMS
- ASSiSTANCE WITH PHASE SELECTION
CLICK ON “List of optimized systems and calculated binary phase diagrams.”
General
The FactSage FScopp copper alloy database is directed primarily to the liquid state of copper-rich alloys, for which a large amount of assessed thermodynamic data is already available. It is based on relevant sub-systems from the old SGTE Solution Database, but now incorporating updates of those systems as well as many new published and in-house assessments. Data compiled by Coursol for the Cu-rich liquid phase [1] have also been incorporated.
While the optimized thermodynamic parameters contained in FScopp are intended primarily to provide a sound basis for calculations relating to copper production and refining, copper-rich solid phases are also included in the database. This makes possible the calculation of liquidus temperatures and solidification characteristics relevant to the casting of certain copper-rich alloys, although, because of the more limited amount of assessed data for solid ternary and higher-order phases available, the results should be treated with caution.
Please note that the FScopp database is a self-consistently evaluated database designed to be used independently of any other. Considerable caution must be exercised if it is used in conjunction with other FactSage alloy databases. However, calculations involving the gas phase can be performed with the FACTPS Database.
The elements included as alloying components of copper are:
Ag, Al, As, Au, Be, Bi, C, Ca, Cd, Co, Cr, Fe, Ga,
Ge, H, Hg, In, Li, Mg, Mn, Nb, Ni, O, P, Pb, Pd,
Pt, S, Sb, Se, Si, Sn, Te, Ti, Tl, V, W, Zn, Zr
In the above list of elements, C, Ca, Ga, Ge, H, Hg, In, Li, Pd, Pt, Tl, V and W are considered minor alloying elements and Gibbs energy model parameters are only available for the FCC-A1, BCC-A2 and Liquid solutions. However, most of the pure compounds of these subsystems are present in the database.
Specific information on each alloy system can be obtained from the list of references supplied further below.
[1] P.Coursol, Report from CRCT, Ecole Polytechnique de Montreal, August 2001.
Composition Ranges
As mentioned above, the database is intended primarily for calculations relating to
Cu-rich liquid alloys. However, some uses may involve relatively large concentrations of the alloying elements present. For this reason, and with the exception of alloys of Cu with Au, Ga, Ge, In, O, P, Pd, Pt, Se and Te, all the copper binary systems are described over all ranges of composition and temperature, i.e. the assessed data provide a good description of the complete phase diagram and thermodynamic properties for the binary alloy system concerned.
Ternary interaction parameters have been assessed for only a few Cu-rich Cu-A-B ternary systems. The number of such assessed parameters is particularly limited in the case of solid phases. Many other ternary interactions in Cu-rich Cu-A-B solutions are estimated, using the appropriate models, from the assessed binary parameters for Cu-free A-B phases. Note that calculation of phase boundaries in higher-order systems may give very unreliable results when the ternary interaction parameters for the solid solutions are estimated by combination of such binary parameters.
Temperature Ranges
The database is generally valid for the temperature range of approximately 400oC to 1600oC.
Modeling
In the assessments, the liquid phase has been described using the Modified Quasichemical Model in the Pair Approximation (MQMPA) which evaluates the effect of short-range order between 2 elements in the solution. Some binary assessments were made equivalent to a Bragg-Williams (random mixing) approximation when the published binary assessment dictated that choice. The fcc Cu-rich phase has been described as a substitutional solid solution and several other non-stoichiometric intermetallic phases have been described using a sublattice model.
Systems assessed
The following matrix is an overview of the binary optimized subsystems in the FScopp Database:
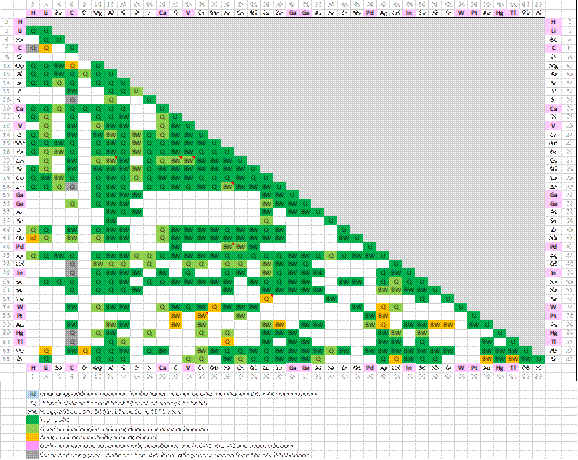
Please note that for the minor alloying elements denoted by the pink color in the matrix, the quality assessment is based only on the FCC-A1, BCC-A2 and Liquid solutions (and the pure compounds) and not on all phases within these binary subsystems.
Phase Selection in the EQUILIB and PHASE DIAGRAM Modules
The database has been constructed in order to simplify the phase selection in the EQUILIB and PHASE DIAGRAM modules of FactSage:
If the database is used alone (i.e. without any other connected databases), you can simply select all pure solids and all solution phases (i.e. click on the button “Select” and the submenu item “Select all solutions”). If the activity of a pure liquid species is intended to be computed, then select all pure liquids.
If the database is used in conjunction with FACTPS, you can simply select all gaseous species and all pure solids. Then select all solution phases (i.e. click on the button “Select” and the submenu item “Add all solutions from database | FScopp”). If the activity of a pure liquid species is intended to be computed, then select all pure liquids. Select carefully the species and solutions from other databases (i.e. FToxid, FTsalt, etc.), however, avoid to include solutions and pure species from another alloy database (i.e. FSstel, FTlite, etc.) as there can be important differences between these databases and FScopp.
In the EQUILIB module for large systems (i.e. more than 5 elements), some solutions will see their default phase selection (“+, I, J, etc.”) changed from “I” (i.e. possible 2-phase immiscibility) to “!” (i.e. dormant (metastable) phase) as these solutions contain a very large number of end-members (hundreds to thousands of phase constituents). For these solutions, no mass can be attributed to them as they are set to be metastable. However, a post-calculation is performed in order to evaluate their activity. If their activity is greater than 1, then it is recommended that you repeat the calculation(s) with the phase selection overridden as “+” or “I” (by right-clicking on the “+”-column (2nd column) of the “Products | Solution phases” box of the MENU Window.
Assessed Binary Copper-containing Systems
Cu-Ag
Cu-Al
Cu-As
Cu-Au (no ordered phases AuCu3-L12, Au3Cu-L12, AuCu-L10)
Cu-Be (CuBe2 approx. a a pure compound)
Cu-Bi
Cu-C
Cu-Ca
Cu-Cd
Cu-Co
Cu-Cr
Cu-Fe
Cu-Ga
Cu-Ge
Cu-H (for P(H2) ≤ 1 atm)
Cu-Hg
Cu-In
Cu-Li
Cu-Mg
Cu-Mn
Cu-Nb
Cu-Ni
Cu-O (for P(O2) ≤ 1.0x10-4 atm)
Cu-P (best for Cu-Cu3P)
Cu-Pb
Cu-Pd
Cu-Pt
Cu-S
Cu-Sb (best for Cu-Cu2Sb)
Cu-Se (best for Cu-Cu2Se)
Cu-Si
Cu-Sn
Cu-Te (best for Cu-Cu2Te)
Cu-Ti
Cu-Tl
Cu-V
Cu-Zn
Cu-Zr
Assessed Ternary interaction parameters in Cu-rich systems
(assessed parameters for certain phases only – click on “List of optimized systems and calculated binary phase diagrams” for details.)
Cu-Ag-Pb
Cu-Al-Mg
Cu-Al-Si
Cu-Al-Sn
Cu-Al-Zn
Cu-As-Pb
Cu-Au-Pb
Cu-Bi-Pb
Cu-Fe-Ni
Cu-Fe-P
Cu-Fe-Pb
Cu-Fe-S
Cu-Mg-Ni
Cu-Mg-Si
Cu-Mg-Y
Cu-Mg-Zn
Cu-Ni-S
Cu-Ni-Si
Cu-P-Sn
Cu-Pb-Sb
Cu-Pb-Sn
Cu-Pb-Zn
Cu-Sn-Zn
Other assessed binary interaction parameters from which ternary interactions in Cu-rich systems are estimated (please refer to the matrix of binary assessed subsystems)
Other assessed ternary interaction parameters from which quaternary interactions in Cu-rich systems are estimated (assessed parameters for certain phases only – click on “List of optimized systems and calculated binary phase diagrams” for details.)
Al-Li-Mg
Al-Li-Si
Al-Mg-Si
Al-Mg-Zn
Al-Si-C
Al-Si-Ca
Al-Si-H
Al-Si-Zn
Al-Sn-Zn
Au-In-Pb
Cr-Fe-Ni
Cr-Fe-V
Fe-Mn-Si
Fe-Ni-P
Use of the Database
The phase diagrams of all the copper-containing binary systems listed above have been checked using FactSage.
References
Pure Element Data
A.T.Dinsdale, SGTE Data for Pure Elements, Calphad 15 (1991) 317-425
Binary Copper-containing Systems
Cu-Ag: Wang, Jian & Cui, Senlin & Rao, Weifeng. (2018). Journal of Electronic Materials. 47.
Cu-Al: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.28-33
Cu-As: M.Hamalainen, private communication
Cu-Au: B.Sundman, S.G.Fries, A.Oates, Calphad 22 (1998) 335-354
Cu-Be: P.J.Spencer, (2003)
Cu-Bi: J. Wang, CRCT (2015)
Cu-C: Shubhank and Y.-B.Kang CALPHAD 45 (2014) 127–137
Cu-Ca: D.Risold, B.Hallstedt, L.J.Gauckler, H.L.Lukas, S.G.Fries, Calphad 20 (1996) 151-160
Cu-Cd: X-M Chen, L-B Liu, L-G Zhang, H. Bo and Z-P Jin Trans Nonferrous Met Soc. China, 20 (2010) 649-654
Cu-Co: P.J.Spencer - L.Kaufman interaction parameters with SGTE element data.
Cu-Cr: S. Cui and I.-H Jung CALPHAD (2017)
Cu-Fe: Shubhank and Y-B. Kang, CALPHAD 2014
Cu-Ga: Li et al., CALPHAD 32(2) (2008), 447-453
Cu-Ge: Wang et al. J.Alloys Cmpds 2010
Cu-H: J.-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Cu-Hg: Y. Liu et al. / Thermochimica Acta 547 (2012) 83–88
Cu-In: C.R.Kao, A.Bolcavage et al, J Phase Equilibria 14 (1993) 22-30
Cu-Li: N Saunders, COST 507 (1998) ISBN 92-828-3902-8 p 168-169
Cu-Mg: S. Cui and I.-H Jung CALPHAD (2017)
Cu-Mn: S. Cui and I.-H Jung CALPHAD (2017)
Cu-Nb: Byeong Joo Lee database, private comunication to SGTE 1999
Cu-Ni: M. Mezbahul-Islam and M. Medraj, “Experimental study of the Cu-Ni-Y system at 700°C using diffusion couples and key alloys” Journal of Alloys and Compounds, 561(5), 161-173 (2013)
Cu-O: P. Chartrand (2018)
Cu-P: P. Chartrand (2018)
Cu-Pb: P. Chartrand (2018)
Cu-Pd: G.K.Sigworth, J.F.Elliott, Canad.Met.Quarterly 13 (1974) 455-461.
Cu-Pt: J.R. Taylor, Platinum Metals Rev. (1985) 29, 2, 74-80
Cu-S: P. Waldner, internal report, CRCT (2005)
Cu-Sb: SGTE Solution Database, 2004.
Cu-Se: P. Chartrand (2018)
Cu-Si: S. Cui and I.-H Jung CALPHAD (2017)
Cu-Sn: J. Wang, internal report, CRCT (2015)
Cu-Te: P. Chartrand (2018) and P.Coursol, Report from CRCT, Ecole Polytechnique de Montreal, August 2001.
Cu-Ti: H.C.Hari Kumar, I.Ansara, P.Wollants, L.Delaey, Z.Metallkde., 87 (1996) 666-672
Cu-Tl: P.Y.Chevalier, Thermochimica Acta 156 (1989) 383-392
Cu-V: Byeong Joo Lee database, private comunication to SGTE 1999
Cu-W : P. Chartrand (2018)
Cu-Zn: Liang, Hsiao, Schmid-Fezter CALPHAD, 2015
Cu-Zr: D.H.Kang I.H.Jung Intermetallics 2010
Ternary Interaction Parameters in Cu-rich Systems– (assessed parameters for certain phases only – click on “List of optimized systems and calculated binary phase diagrams” for details.)
Cu-Ag-Pb:
Cu-Al-Mg:
Cu-Al-Si:
Cu-Al-Sn:
Cu-Al-Zn:
Cu-Fe-Ni:
Cu-Fe-P:
Cu-Mg-Ni:
Cu-Mg-Si:
Cu-Mg-Zn:
Cu-Ni-Si:
Cu-Pb-Zn:
Cu-Sn-P:
Cu-Sn-Zn:
Other Binary Parameters (assessed parameters for certain phases only – click on “List of optimized systems and calculated binary phase diagrams” for details.)
Ag-Al: S.S.Lim, P.L.Rossiter, J.W.Tibbals, Calphad 19 (1995) 131-142
Ag-As: P. Chartrand (2018)
Ag-Au: Re-assessed MQMPA liquid from S.Hassam, J.Agren, M.Gaune-Escard, J.P.Bros, Met.Trans. 21A (1990) 1877-1884
Ag-Bi: Wang, Jian & Cui, Senlin & Rao, Weifeng. (2018). Journal of Electronic Materials. 47.
Ag-C: P. Chartrand (2014)
Ag-Ca: J. Wang, P. Chartrand and I-H Jung, CALPHAD 50 (2015)
Ag-Co: P. Chartrand (2014)
Ag-Fe: P. Chartrand (2014)
Ag-Ga: W. Gierlotka, D. Jendrzejczyk-Handzlik, Journal of Alloys & Compounds 509 (2011) 38
Ag-Ge: P.Y.Chevalier, E.Fischer, private Communication, 1998:
Ag-H : P. Chartrand (2014)
Ag-Hg: Y. Liu et al. / Thermochimica Acta 547 (2012) 83–88
Ag-In: J. Wang, P. Hudon, D. Kevorkov, P. Chartrand, I-H Jung, M. Medraj, JPE 2014, 35(3), 284-313
Ag-Li: J. Wang, P. Chartrand and I-H Jung, CALPHAD 50 (2015)
Ag-Mg: J. Wang, P. Chartrand and I-H Jung, CALPHAD 50 (2015)
Ag-Mn: I. Karakaya, W. T. Thompson, Bull. Alloy Phase Diagrams, 1990, 11, (5), 80-486
Ag-Ni: Wang, Jian & Cui, Senlin & Rao, Weifeng. (2018). Journal of Electronic Materials. 47.
Ag-P: P. Chartrand (2018)
Ag-Pb: H.-L. Lukas, 1998
Ag-Pd: G. Ghosh, C. Kantner, G. B. Olson, J. Phase Equilib., 1999, 20(3), 295-308
Ag-S: P. Chartrand (2018)
Ag-Sb: E. Zoro, C. Servant, B. Legendre, Journal of Phase equilibria and Diffusion, 2007, 28, 250-257
Ag-Se: P. Chartrand (2018)
Ag-Si: S.Hassam, J.Agren, M.Gaune-Escard, J.P.Bros, Met.Trans. 21A (1990) 1877-1884
Ag-Sn: J. Wang, P. Hudon, D. Kevorkov, P. Chartrand, I-H Jung, M. Medraj, JPE 2014, 35(3), 284-313
Ag-Ti: Mei Li, Changrong Li, Fuming Wang, Weijing Zhang, CALPHAD, 2005, 29, 269-275
Ag-Tl: H.-L. Lukas, reassessment based on of Zimmerman’s thesis, 1976
Ag-W: P. Chartrand (2018)
Ag-Zn: J. Wang, Y-N Zhang, P. Hudon, I-H Jung, M. Medraj, P. Chartrand, J. Alloys Cmpds (2015) 639, p. 593
Ag-Zr: D.H. Kang, I.H. Jung, Intermetallics 18 (2010) 815-833
Al-As: P.J. Spencer (2008)
Al-Au: J.Murray, H.Okamoto, T.B.Massalski, Bull.Alloy Phase Diags.8 (1987) 20-30
(Modified by A.T.Dinsdale to give compatibility with SGTE unary data and to prevent
high-temp. stability of fcc)
Al-Be: M. Piché, Master Thesis, Polytechnique Montreal (2002)
Al-Bi: M. Paliwal (2009)
Al-C: P. Chartrand, CRCT (2004)
Al-Ca: Y-B Kang, CRCT (2008)
Al-Co: Based on N.Dupin, I.Ansara, Rev. de Met. 9 (1998) 1121-1129
Al-Cd: P. Chartrand (2017)
Al-Co: N. Dupin, I. Ansara, La Revue de Metallurgie-CIT/Science et Genie des Materiaux, September 1998, 1121-1129
Al-Cr: P. Chartrand (2006) MQMPA refitted from N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, 23-27; based on: Z Metallkde. 78 (1987) 795-801
Al-Fe: A.T. Phan & Y-B Kang, Acta Materiala 2014, modifications from P. Chartrand (2018)
Al-Ga: P. Chartrand (2018) MQMPA refitted from A.Watson CALPHAD 1992
Al-Ge: I. Ansara, J. P. Bros, M. Gambino, CALPHAD 1979, 3(3), 225
Al-H: J.-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Al-Hg: A. J. McAlister, Bull. Alloy Phase Diagrams, 1985, 6, (3), 219-221
Al-In: I. Ansara, C Chatillon, H. L. Lukas, T. Nishizawa, H. Ohtani, K. Ishida, M. Hillert, B. Sundman, B. B. Argent, A. Watson, T. G. Chart, T. Anderson, CALPHAD 1994, 18(4), 177-222
Al-Li: J.-P. Harvey, CRCT (2008)
Al-Mg: P.Chartrand (2006)
Al-Mn: Min-Su Kim and Y-B Kang, JPED 2015
Al-Nb: C Servant and I. Ansara, J. Chim. Phys. 1997, 94, 869-888
Al-Ni: I.Ansara, N.Dupin, H.-L. Lukas, B.Sundman, J.Alloys and Cpds. 247 (1997) 20-30
Al-O: P. Chartrand (2003)
Al-P: P. Chartrand (2014)
Al-Pb: Y-B. Kang (2009)
Al-S: P. Chartrand (2007) MQMPA refit from R.C.Sharma and Y.A.Chang BAPD, 8(2) 1987 p.128-131
Al-Sb: M. Paliwal, Master Thesis, McGill Univ. (2009)
Al-Se: P. Chartrand (2018)
Al-Si: J.-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Al-Sn: Y-B. Kang and A.D. Pelton, CALPHAD 34 (2010)
Al-Ti: P. Chartrand (2006) MQMPA refit from N Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.89-94
Al-Tl: P. Chartrand (2018) MQMPA refit from McAlister 1989 BAPD
Al-V: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.95-98
Al-W: COST 507 (1998)
Al-Zn: P. Chartrand (2006) MQMPA refit from S.an Mey, Z.Metallkde. 84 (1993) 451-455
Al-Zr: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.112-116
As-Au: P.J.Spencer, June 1998
As-Bi: P. Chartrand (2018)
As-Fe: P.J. Spencer (2008)
As-Ga: C Chatillon, I. Ansara, A. Watson and B. B. Argent: CALPHAD, 1990, 14(2), 203-14.
As-Ge: I. Ansara and D. Dutarte: CALPHAD, 1984, 8(4), 323-342.
As-In: C.Chatillon, I.Ansara, A.Watson, B.B.Argent, Calphad 14 (1990) 203-214
As-P: I Ansara, C Chatillon, H. L. Lukas, T. Nishizawa, H. Ohtani, K. Ishida, M. Hillert, B. Sundman, B. B. Argent, A. Watson, T. G. Chart, T. Anderson: CALPHAD 1994, 18(4), 177-222.
As-Pb: M.Hamalainen, private communication
As-Sb: H.Ohtani, Calphad 18 (1994) 196
As-Si: P.J. Spencer (2006)
As-Te: P. Chartrand (2018)
Au-Bi: P.Y.Chevalier, Thermochimica Acta 136 (1988) 15-24
Au-C : P.J. Spencer (2007)
Au-Cr: P.J. Spencer, (1998)
Au-Ge: P. Y. Chevalier: Thermochimica Acta, 1989, 141, 217-226.
Au-In: I.Ansara, J.P.Nabot, Calphad 16 (1992) 13-18
Au-Pb: J.P.Nabot, Thesis, Grenoble,1986
Au-Pd: COST-531
Au-Sb: P.Y.Chevalier, Thermochimica Acta 155 (1989) 211-225
Au-Si: P.Y.Chevalier, private communication to SGTE, July 1998
Au-Sn: P.Y.Chevalier, Thermochimica Acta 130 (1988) 1-13
Au-Te : Y. Feutelais, D. Mounai, J. R. Didry, B. Legendre: J. Phase Equil., 1994, 15(4), 380-385. (with modified AuTe2)
Au-Ti: P.J.Spencer, July 1998
Au-Tl: P.Y.Chevalier, Thermochimica Acta 155 (1989) 211-225
Au-Zn : COST-531
Be-Ca : P. Chartrand (2014)
Be-Li : P. Chartrand (2014)
Be-Fe : M. Piché, Master Thesis, Polytechnique Montreal (2002)
Be-Mg : M. Piché, Master Thesis, Polytechnique Montreal (2002)
Be-Mn : M. Piché, Master Thesis, Polytechnique Montreal (2002)
Be-Si: P. Chartrand (2014)
Be-Sn: P. Chartrand (2014)
Be-Zn: P. Chartrand (2014)
Bi-Cd: P. Chartrand (2014)
Bi-Co: P. Chartrand (2014)
Bi-Cr: P. Chartrand (2014)
Bi-Fe: Li-Mei Pan, unpublished research (1991)
Bi-Ga: C. Girard: Thesis, Marseille 1985.
Bi-Ge: P. Y. Chevalier: Thermochimica Acta, 1988, 132, 111-116
Bi-Hg: unpublished assessment of S. A. Mucklejohn.
Bi-In: D.Boa, I.Ansara, Thermochimica Acta 314 (1998) 79-86
Bi-Li: Z. Cao, Xie, Chartrand, Wei, CALPHAD 46 (2014)
Bi-Mg: M. Paliwal, Master Thesis, McGill Univ. (2009)
Bi-Ni: J. Wang, CRCT (2015)
Bi-Pb: D.Boa, I.Ansara, Thermochimica Acta 314 (1998) 79-86
Bi-S: P. Chartrand (2018)
Bi-Sb: P. Chartrand (2014) MQMPA refit from H.Ohtani, K.Ishida, J.Electronic Mater. 23 (1994) 747-755
Bi-Si: P.J. Spencer (2008)
Bi-Sn: J. Wang, CRCT (2015)
Bi-Tl: Zimmermann B., Henig E. T., Lukas H. L.: Z. Metallkde., 1976, 67(12), 815-820
Bi-V: P. Chartrand (2014)
Bi-Zn: C.Girard, Thesis, Marseille, 1985
C-Ca : P. Chartrand (2018)
C-Co : A. Fernandez Guillermet: Z. Metallkde., 1987, 78, 700-9
C-Cr : B. J. Lee: CALPHAD 1992, 16(2), 121-149
C-Fe: M-S Kim, Y-B Kang JPE 2015
C-Ge: P. Chartrand (2014)
C-Mg: P. Chartrand (2014)
C-Mn: M.K. Paek, Y-B Kang, CALPHAD 46 (2014), 92-102
C-Nb: WM Huang: Mater. Sci. and Techn. 1990, 6(8), 687-694
C-Ni: B. J. Lee: CALPHAD, 1992, 16(2), 121-149.
C-P : P. Gustafson: Inst. Met. Res. (Report IM-2549, 1990))
C-Pb : unpublished assessment of T. G. Chart, NPL 1987
C-Sb: P. Chartrand (2018)
C-Si: M-K Paek, Y-B Kang CALPHAD 46 (2014) 92–102
C-Sn: P. Chartrand (2007)
C-Ti: P. J Spencer (2008)
C-V: WM Huang : Z. Metallkde, 1991, 82, (3), 174-181
C-W: P. Gustafson: Report TRITA 0212 (1985), Mat. Sci and Tech. 1986, 2(7), 653-658
C-Zr: A. Fernandez Guillermet: J. Alloys Compounds, 1995, 217, 69-89.
Ca-Co : P. Chartrand (2014)
Ca-Cr : P. Chartrand (2014)
Ca-Fe: S.Cui, M.Paliwal and I.-H. Jung, MetTrans 2014
Ca-H: J.-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Ca-In: J. Wang, P. Chartrand and I-H Jung, CALPHAD 50 (2015)
Ca-Li: J. Wang, P. Chartrand and I-H Jung, CALPHAD 50 (2015)
Ca-Mg: P.-A. Anctil, Polytechnique Montreal (2003)
Ca-Mn : P.J Spencer (2006)
Ca-Nb: P. Chartrand (2016)
Ca-Ni: M. Medraj (2006)
Ca-O: P. Chartrand (2000)
Ca-Pb: V.P.Itkin and C.B.Alcock, J. Phase Equilib. 1992, pp.162-169
Ca-Si: M. Heyrmann and P. Chartrand, JPE (2005)
Ca-Sn: J. Wang, Ph.D. Thesis, Polytechnique Montreal (2014)
Ca-Ti: P. Chartrand (2014)
Ca-V: P. Chartrand (2014)
Ca-W: P. Chartrand (2014)
Ca-Zn: P.J. Spencer (2006)
Ca-Zr: P. Chartrand (2014)
Cd-Co: P. Chartrand (2018)
Cd-Cr: P. Chartrand (2018)
Cd-Fe: P. Chartrand (2018)
Cd-Ga: Zakulski W., Moser Z., Rzyman K., Lukas H. L., Fries S. G., Sikiennik M., Kaczmarczyk R., Castanet R.: J. Phase Equil., 1993, 14(2), 184-196.
Cd-Ge: P. Chartrand (2018)
Cd-Hg: Jang J., Silk N. J., Watson A., Bryant A. W., Chart T. G., Argent B. B.: CALPHAD, 1995, 19(3), 415-430
Cd-In: Zakulski W., Moser Z., Rzyman K., Lukas H. L., Fries S. G., Sikiennik M., Kaczmarczyk R., Castanet R.: J. Phase Equil. 1993,14(2),184-196
Cd-Mg: Ren, Li, Guo, Du, Thermochimica Acta 2012 (without ordered phases)
Cd-Pb: W. Zakulski, Z. Moser: J. Phase Equilib, 1995, 16(3), 239-242. W. Zakulski, Z. Moser: J. Phase Equilib, 1995, 16(6), 484.
Cd-S: P. Chartrand (2018)
Cd-Sb: L. A. Zabdyr: CALPHAD 1997, 21(3), 349-358.
Cd-Si: P. Chartrand (2018)
Cd-Sn: P. Chartrand (2018)
Cd-Tl : Y. Liu et al. / Journal of Alloys and Compounds 473 (2009) 60–64
Cd-V: P. Chartrand (2018)
Cd-W: P. Chartrand (2018)
Cd-Zn: L. A. Zabdyr: CALPHAD 1997, 21(3), 349-358.
Co-Cr: A.Kusoffsky, B.Jansson, Calphad 21 (1997) 321-333
Co-Fe: A.F.Guillermet, High Temp. High Press. 19 (1988) 477-499
Co-In: D. Boa, B. K. Dongui, I. Ansara: CALPHAD 25 (2001) 645-650
Co-Li: P. Chartrand (2014)
Co-Mg: P. Chartrand (2018)
Co-Mn: W.Huang, Calphad 13 (1989) 231-242
Co-Nb: K.C.H.Kumar, I.Ansara, P.Wollants, L.Delaey, J.Alloys and Cpds. 267 (1998) 105-112
Co-Ni: A.F.Guillermet, Z.Metallkde. 78 (1987) 639-647; Z.Metallkde. 79 (1988) 524-536
Co-S: P. Waldner, A.D. Pelton, internal report, CRCT (2004)
Co-Si: S.D.Choi, Calphad 16 (1992) 151-159.
Co-Ti: G.Cacciamani, R.Ferro, I.Ansara, N.Dupin, submitted to "Intermetallics", 1999
Co-V: J. Bratberg, B. Sundman: J. Phase Equil., (2003), 24(6), 495-503
Co-W: Markstrom, Sundman & Frisk JPE (2005)
Co-Zn: G.P. Vassilev, M. Jiang: J. Phase Equil. and Diffusion 2004, 25, 259-268
Co-Zr: Durga & Kumar, CALPHAD 2010
Cr-Fe: Cui & I.-H. Jung CALPHAD 2017
Cr-H: J.-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Cr-Hg: P. Chartrand (2018)
Cr-Li: P. Chartrand (2003)
Cr-Mg: I. Ansara, COST 507 (1998) ISBN 92-828-3902-8, p.143-144
Cr-Mn: B.J. Lee, Metall.Trans.24A (1993) 1919
Cr-Nb: J.G.Costa Neto, S.G.Fries, H.-L.Lukas, Calphad 17 (1993) 219-228
Cr-Ni: B.J.Lee, Calphad 16 (1992) 121-149
Cr-P: NPL, unpublished work, 1989
Cr-Pb: P. Chartrand (2003)
Cr-Pt: Oikawa et al. Journal of Magnetism and Magnetic Materials 236 (2001) 220–233
Cr-S: P. Waldner, A.D. Pelton, internal report, CRCT (2004)
Cr-Si: S Cui & In-Ho Jung, Met.Trans.2017
Cr-Sn: R. Jerlerud Perez, B. Sundman: CALPHAD, 25 (2001) 59-66.
Cr-Ti: N.Saunders, COST 507 (1994) ISBN 2-87263-156-9, p.103
Cr-V: B.J.Lee, Z.Metallkde 83 (1992) 292-299
Cr-W: P. Gustafson: Report TRITA-MAC 320 (1986), CALPHAD, 1988, 12(3), 277-292.
Cr-Zn: I.Ansara, COST 507 (1998) ISBN 92-828-3902-8, p.158-160
Cr-Zr: K.Zeng, M.Hamalainen, I.Ansara, COST 507 (1998) ISBN 92-828-3902-8 p 161-164
Fe-H: J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Fe-Hg: P. Chartrand (2018)
Fe-In: P. Chartrand (2018)
Fe-Li: P. Chartrand (2003)
Fe-Mg: P. Chartrand (2006)
Fe-Mn: M-S Kim, Y-B Kang, JPE, (2015)
Fe-Nb: Khvan & Hallstedt CALPHAD (2013)
Fe-Ni: A.Dinsdale, T.Chart, NPL, unpublished work, 1986: I.Ansara - fcc ordering
Fe-P: P. Chartrand (2018)
Fe-Pb: P. Chartrand (2018)
Fe-S: P. Waldner and A.D.Pelton, "Thermodynamic Assessment of the Fe-Ni-S System", Metall. and Mat. Trans., 35B, 897-907 (2004).
Fe-Sb: P.J.Spencer, 1998
Fe-Si: S Cui & In-Ho Jung, Met.Trans.2017
Fe-Sn: K.C.H.Kumar, P.Wollants, L.Delaey, Calphad 20 (1996) 139-149 (with modifs from P. Chartrand (2018))
Fe-Ti: L.F.S.Dumitrescu, M.Hillert and N.Saunders, J.Phase Equilibria 19 (1998) 441-448
Fe-Tl: P. Chartrand (2018)
Fe-V: W.Huang, Z.Metallkde 82 (1991) 391-401
Fe-W: J-O Andersson and P Gustafson: CALPHAD, 1983, 7(4), 317-326.
Fe-Zn: P. Chartrand (2018) MQMPA calibrated on the solids of Xiong, Du Liu CALPHAD 2009
(later modifications by M.Jacobs)
Fe-Zr: M. Bejarano, internal report CRCT (2009)
Ga-Ge: I Ansara, J P Bros, M Gambino: CAPHAD, 1979, 3, 225-233
Ga-Hg: unpublished assessment of I. Ansara, (1991).
Ga-In: B. C. Rugg, T. G. Chart: CALPHAD, 1990, 14(2), 115-123
Ga-Mg: Y.-B. Kang, CALPHAD 2014
Ga-P: I. Ansara, C. Chatillon, H. L. Lukas, T. Nishizawa, H. Ohtani, K. Ishida, M. Hillert, B. Sundman, B. B. Argent, A. Watson, T. G. Chart, T. Anderson: CALPHAD, 1994, 18(4), 177-222
Ga-Pb: I. Ansara, F. Ajersch: J. Phase Equil., 1991, 12(1), 73-77
Ga-Sb: I. Ansara, C. Chatillon, H. L. Lukas, T. Nishizawa, H. Ohtani, K. Ishida, M. Hillert, B. Sundman, B. B. Argent, A. Watson, T. G. Chart, T. Anderson: CALPHAD, 1994, 18(4), 177-222
Ga-Si: Olesinski BAPD (6) 1985, 362-364
Ga-Sn: T. J. Anderson, I. Ansara: J. Phase Equilibria, 1992, 13(2), 181-189
Ga-Tl: I. Katayama et al., T. Iida, Z. Metallknd. 94, 2003, p.1296
Ga-Zn: Dutkiewicz, J., Moser, Z., Zabdyr, L., Gohil ,D. D., Chart, T. G., Ansara I., Girard, C.: Bull. Alloy Phase Diagrams, 1990, 11(1), 77-82
Ge-In: P. Y. Chevalier: 1989, 155, 227-240
Ge-Mg : I-H Jung et al. J. Alloys and Cmpds (2010)
Ge-Pb: P. Chevalier, Thermochimica Acta, 1989, vol 155, pp. 227-240
Ge-Sb: P. Y. Chevalier, Thermochimica Acta, 1989, 155, 227-240.
Ge-Si : Z. H. Long, H.S. Liu, Z.P. Jin: J. Alloys Compounds, 479 (2009) 262-267
Ge-Sn: Y. Feutelais, B. Legendre, S. G. Fries: CALPHAD, 1996, 20(1), 109-123
Ge-Tl: P. Chevalier, Thermochimica Acta, 1989, vol 155, pp. 227-240
Ge-Zn: P. Chevalier, Thermochimica Acta, 1989, vol 155, pp. 227-240
H-Li : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Mg : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Mn : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Ni : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Si : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Ti : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Zn : J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
H-Zr : (H-Ti) from J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Hg-Mg: P. Chartrand (2018)
Hg-Pb: A. Maitre, J. M. Fiorani, M. Vilasi: J. Phase Equilib., 2002, 23(4), 329
Hg-S : P. Chartrand (2018)
Hg-Sn: Yee-Wen Yen, Joachim Grobner, Steve C. Hansen, and Rainer Schmid-Fetzer JPE, 24(2), p.151-167, 2003
Hg-Zn: S. C. Hansen: CALPHAD, 1998, 22, 359-373.
In-Mg : Wang, J., Hudon, P., Kevorkov, D. et al. J. Phase Equilib. Diffus. (2014) 35: 284
In-P: I.Ansara, C.Chatillon, Calphad 18 (1994) 204
In-Pb: D.Boa, I.Ansara, Thermochimica Acta 314 (1998) 79-86
In-Sb: T.J.Anderson, Calphad 18 (1994) 206
In-Si: R.W.Olesinski, N.Kanani, G.J.Abbaschian, Bull.Alloy Phase Diags.6 (1985) 128-130
In-Sn : Wang, J., Hudon, P., Kevorkov, D. et al. J. Phase Equilib. Diffus. (2014) 35: 284
In-V: P. Chartrand (2014)
In-Zn : Wang, J., Hudon, P., Kevorkov, D. et al. J. Phase Equilib. Diffus. (2014) 35: 284
Li-Mg: P.J Spencer & J. Wang, CRCT (2008, 2011)
Li-Mn: Y-B. Kang, internal report CRCT (2005)
Li-Nb: P. Chartrand (2016)
Li-Ni: P. Chartrand (2003)
Li-Pb: P. Chartrand (2003)
Li-Si: J.-P. Harvey and P. Chartrand (2007)
Li-Sn: J. Wang, Ph.D. Thesis, Polytechnique Montreal (2011)
Li-Ti: P. Chartrand (2014)
Li-V: P. Chartrand (2014)
Li-Zn: P.J. Spencer, internal report, CRCT (2006)
Li-Zr: P. Chartrand (2014)
Mg-Mn: Y-B Kang, internal report, CRCT (2005)
Mg-Nb: P. Chartrand (2014)
Mg-Ni: M.Jacobs, COST 507 report (1998) ISBN 92-828-3902-8, p.218-220
Mg-Pb: D. Nassyrov, I.-H. Jung, CALPHAD, 33 (2009) 521-529
Mg-Sb: M. Paliwal, I.-H. Jung, CALPHAD, 33 (2009) 744-754.
Mg-Si: J-P. Harvey, Master Thesis, Polytechnique Montreal (2006)
Mg-Sn: J. Wang, P. Hudon, D. Kevorkov, P. Chartrand, JPE 35 (2014)
Mg-Ti: CRCT internal report (2008)
Mg-V: P. Chartrand (2014)
Mg-W: P. Chartrand (2014)
Mg-Zn: P.J. Spencer, internal report, CRCT (2006)
Mg-Zr: Bejarano, CRCT internal report (2009)
Mn-Nb: Liu & Hallstedt CALPHAD 2012
Mn-Ni: NPL, unpublished work, 1989
Mn-P: unknown
Mn-Pb: A.T.Dinsdale, D.D.Gohil, NPL, unpublished work, 1987
Mn-Si: M.K. Paek, Y-B Kang, CALPHAD 46 (2014)
Mn-Sn: J. Miettinen: CALPHAD, 2001, 25(1), 43-58.
Mn-Ti: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.241-244
Mn-V: W.Huang, Metall.Trans. 22A (1991) 1911-1920
Mn-W: P. Chartrand (2018)
Mn-Zn: Y-B Kang, internal report, CRCT (2005)
Mn-Zr: K.Hack, COST 507 (1998) ISBN 92-828-3902-8, p.245-248
Nb-Ni: Joubert, Dupin, Sundman CALPHAD 2004
Nb-Si: H. Liang and Y. A. Chang: Intermetallics, 1999, 7, 561-570.
Nb-Ti: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.256-260
Nb-V: K.C.H.Kumar, P.Wollants, L.Delaey, Calphad 18 (1994) 71-79
Nb-W: Weiming Huang, M. Selleby: Z. Metallkde., 1997, 88(1), 55-62
Nb-Zn: Z.Long JPE 2016
Nb-Zr: A.F.Guillermet, Z.Metallkde 82 (1991) 478-487
Ni-P: NPL, unpublished work, 1989
Ni-Pb: Cui Ping Wang, Xing Jun Liu, I. Ohnuma, R. Kainuma, K. Ishida: CALPHAD, 2000, 24(2), 149-167
Ni-S: P. Waldner and A.D. Pelton, "Thermodynamic Modeling of the Ni-S System", Z. Metallkunde, 95, 672-681 (2004).
Ni-Si: M.Lindholm, B.Sundman, Metall.Trans. 26A (1996) 2897-2903
Ni-Sn : COST-531
Ni-Ti: C.S.Oh, J.Korean Inst.Met.Mater. 33 (1995) 129-136
Ni-V: J.Korb, K.Hack, COST 507 (1998) ISBN 92-828-3902-8, p.261-263
Ni-W: P. Gustafson, A. Gabriel, I. Ansara: Report TRITA 0263(1985), Z. Metallkde, 1986, 78, 151-156).
Ni-Zn: J. Miettinen: CALPHAD, 2003, 27, 263-274
Ni-Zr: G Ghosh, J.Mater.Res. 9 (1994) 598-616 (amended version)
O-Pb: P. Chartrand (2018) – O solubility in Pb
P-Sb: I.Ansara, C.Chatillon, Calphad 18 (1994) 208
P-Si: P.J Spencer (2006)
Pb-Pd : G. Ghosh: J. Phase Equil, 1999, 20(3), 309-315
Pb-S: P. Chartrand (2018)
Pb-Sb: H.Ohtani, K.Okuda, K.Ishida, J.Phase Equilibria 16 (1995) 416-429
Pb-Se: P. Chartrand (2018)
Pb-Si: R.W.Olesinski, G.J.Abbaschian, Bull.Alloy Phase Diags. 5 (1984) 271-273
Pb-Sn: Based on H.Ohtani, K.Okuda, K.Ishida, J.Phase Equilib.16 (1995) 416-429
Pb-Te: P. Chartrand (2018)
Pb-Tl: unpublished work from I. Ansara, H.L. Lukas and S. G. Fries (comm. P.J. Spencer)
Pb-Zn: T.Jantzen, P.J.Spencer, Calphad 22 (1998) 417-434
Pb-Zr: Dixon P. R., Argent B. B., Chart T. G.: CALPHAD, 1998, 22(3), 397-416
Pd-Sn: G. Ghosh: Metall. Mater. Trans. A, 1999, 30A, 5-18
Pd-Ti: K.Hack (1996), based on J. Murray, Bull.Alloy Phase Diagrams 3 (1982) 329.
S-Sb: P. Chartrand (2018)
S-Sn: P. Chartrand (2018)
S-Te: P. Chartrand (2018)
S-Zn: P. Chartrand (2018)
Sb-Si: P.J. Spencer (2006)
Sb-Sn: C.S.Oh, J.H.Shim, B.J.Lee, D.N.Lee, J.Alloys and Cpds. 238 (1996) 155-166
Sb-Zn: L.A.Zabdyr, Calphad 21 (1997) 349-358
Se-Te: G. Ghosh, R. C. Sharma, D. T. Li, Y. A. Chang: J. Phase Equil., 1994, 15(2), 213-224
Si-Sn: M.H.G.Jacobs, P.J.Spencer, Calphad 20 (1996) 89-91
Si-Ti: H.Seifert, COST 507 (1998) ISBN 92-828-3902-8, p.266-269
Si-Tl: P. Chartrand (2018)
Si-V: M.H.Rand, COST 507 (1994), ISBN 2-87263-156-9, p.182
Si-W : P.Y. Chevalier, E. Fischer, Thermodata report, June 2003
Si-Zn: A. Shukla, internal report CRCT (2006)
Si-Zr: C.Gueneau, C.Servant, I.Ansara, N.Dupin, Calphad 18 (1994) 319-327
Sn-Te: P. Chartrand (2018)
Sn-Ti: F.Hayes, COST 507 (1998) ISBN 92-828-3902-8, p.284-287
Sn-Tl: P. Chartrand (2018)
Sn-V: Chen, Gierlotka, and Chen, Journal of Electronic Mat., Vol. 37, No. 11, 2008
Sn-Zn: P Ghosh, MD Mezbahul-Islam, M Medraj - Calphad, 2012
Sn-Zr: J.Korb, K.Hack, COST 507 (1998) ISBN 92-828-3902-8, p.290-292
Ti-V: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.297-298
Ti-W: N.Saunders, COST 507 (1998) ISBN 92-828-3902-8, p.297-298
Ti-Zn: K. Doi, S. Ono, H. Ohtani, M. Hasebe: J. Phase Equilib. Diff., 2006, 27(1), 63-74.
Ti-Zr: H.Kumar, P.Wollants, L.Delaey, J.Alloys and Compounds, (1994) p.121-127
V-W: MQMPA fit of J. Bratberg: Z. Metallkd., 96 (2005) 335-344.
V-Zn: P. Chartrand (2014)
V-Zr: J.Korb, K.Hack, COST 507 (1998) ISBN 92-828-3902-8, p 303-304.
Other Ternary Parameters (assessed parameters for certain phases only – click on “List of optimized systems and calculated binary phase diagrams” for details.)
…upcoming