INTRODUCTION / SUMMARY
The FTOxCN thermodynamic database was developed by the FACT group for performing equilibrium calculations in the Al-(Si-Ca-Mg-Fe-Na)-C-O-N-S system at very high temperatures. Carbonates, cyanides, nitrates, oxidized states of sulfur (e.g. SO42-, S2O72-, etc.) and polysulfides (e.g. S22-, S32-, S42-, etc.) are assumed not to be stable in the high-temperature relatively reducing conditions and are neglected. When Fe is present, the database is only valid for reducing conditions. Even though some technologically important sub-systems have been optimized over their entire composition range (see below), calculations in the 10-component system are most accurate when Al, C and O are the major components and Si, Ca, Mg, Na, Fe, N and S are minor components as a consequence of the relative availability of experimental data. The optimizations will be described in future publications.
The FTOxCN solution database contains oxycarbonitride solutions and a compatible liquid metallic solution. In particular, the liquid "Slag" phase is treated as a single solution phase containing all 10 elements, valid at all temperatures and over all composition ranges of interest. This phase thus incorporates the high-temperature oxycarbide slag, sulfide-rich liquid and oxide slags which might appear at lower temperatures, oxynitride liquids, etc., all in one solution (with possible immiscibility gaps, of course). However, the "Slag" phase does not include the liquid metallic solution which is a separate phase in the FTOxCN solution database. The FTOxCN compound database contains all stoichiometric solid and liquid oxycarbonitride, sulfide and oxysulfide compounds evaluated/optimized to be thermodynamically consistent with the FTOxCN solution database.
Choice of species when using the new database:
The FTOxCN database should be used in combination with the following databases:
- FToxid (for solid oxide solutions and stoichiometric compounds)
- FTlite (for solid metallic solutions and stoichiometric compounds, except for the Al4C3 solution)
- FactPS (for gaseous species)
The liquid phases (Slag and Liquid metal) should always be selected from the FTOCNS solution database. One should NOT use the slag solution from the FToxid database nor a liquid metallic solution from the FTlite or any other database.
The solid solutions should be selected from the FTOxCN, FToxid and FTlite solution databases simultaneously. Note that the Al4C3 solution should be taken from FTOCNS, not from FTlite.
For stoichiometric compounds, the “suppress duplicates” option should be used with the following compound database priority list (highest priority first): FTOxCN FToxid FTlite FactPS. Normally, no solid compounds should be selected from FactPS. Do not select stoichiometric liquids from any database; use solutions slag and liquid metal from FTOxCN instead.
The gaseous species should be selected from FactPS.
Some points to note:
It may be necessary to use the “I” (or possibly even the “J”) option when using the Slag phase, as well as with the AlON spinel phase and the beta-sialon phase. When in doubt, use the “I” or “J” option.
Some of the calculated phase diagrams lie exactly on stoichiometric (that is, pseudobinary or pseudoternary) sections. Consequently, the PhaseDiagram or Equilib modules of FactSage may fail to converge if you try to reproduce these figures. To avoid this problem, add a very small excess of metal, or a very small excess of carbon, or a very small amount of argon (such as 1.0 X 10-4 of the total amount of material) to the input. Convergence should not be a problem in calculations for industrial processes, because such calculations will never lie exactly on a stoichiometric join.
SYSTEMS AND COMPONENTS
The FTOxCN databases contain data for oxycarbonitride solutions and stoichiometric compounds in the Al-(Si-Ca-Mg-Fe-Na)-C-O-N-S system. Not all sub-systems have been evaluated and optimized, nor are all composition ranges covered. Experimental information on oxycarbonitride systems at very high temperatures is very limited and often contradictory. All experimental data for sub-systems that are deemed important for industrial applications have been taken into account. The sub-systems and composition ranges which have been evaluated and optimized are described in the following. The most accurate calculations will be obtained in or near these sub-systems and composition ranges.
The Modified Quasichemical Model in the quadruplet approximation is used for the “Slag” phase. This model assumes that Al3+, Si4+, Mg2+, Ca2+, Fe2+ and Na+ ions are distributed on the sites of a cationic sublattice while O2-, N3-, C4- and S2- ions are distributed on an anionic sublattice. Short-range-ordering between first- and second-nearest neighbors is taken into account in the model.
Carbide Systems
Binary Systems
The Al-C, Si-C and Fe-C systems have been optimized. For optimized phase diagrams, click on “phase diagrams” in this menu.
No data were found for the Mg-C and Ca-C systems. The liquid phases were assumed to be ideal solutions of Ca(liq) or Mg(liq) and C(liq).
For temperatures above 1200 °C, the equilibrium in the Na-C system will simply consist of C(graphite) + gas. The solid solubility of Na in graphite is neglected as are the low-temperature intercalation compounds for which the thermodynamic stability is uncertain. For optimized phase diagrams, click on “phase diagrams” in this menu.
Ternary Systems
The Al-Si-C system has been optimized.
The thermodynamic description of the Al-Fe-C metallic liquid is believed to be well predicted from the binary parameters.
No data were found for the Al-Mg-C, Al-Ca-C and Al-Na-C systems.
Oxycarbide Systems
Ternary Systems
The Al-O-C system has been completely re-evaluated and re-optimized.
In the Si-O-C system, no experimental phase diagram data were found for the SiO2-SiC section. For the SiO2-SiC liquid phase, a large positive excess term was estimated.
In the Mg-O-C, Ca-O-C, Fe-O-C and Na-O-C systems, the hypothetical liquids AO-A2C (involving C4- anions; A = Mg2+, Ca2+, Fe2+, Na+) were assumed ideal.
Reciprocal Oxycarbide Systems
For slags rich
in CaO and MgO, some carbon may be present as acetylide ![]() ions since the compounds
CaC2 and MgC2 are known to be stable at high
temperatures. This is not considered in the present model because the FTOxCN
database is designed for use only at very high temperatures where acetylide
ions are relatively unstable.
ions since the compounds
CaC2 and MgC2 are known to be stable at high
temperatures. This is not considered in the present model because the FTOxCN
database is designed for use only at very high temperatures where acetylide
ions are relatively unstable.
For calculated isothermal sections Al2O3-C-SiO2, Al2O3-C-FeO, Al2O3-C-MgO, Al2O3-C-CaO and Al2O3-C-Na2O at 1 atm total pressure, click on “phase diagrams” in this menu.
Note that in these phase diagram calculations, a very small amount of argon (10-6 moles) was included as a reactant in order that a gas phase always be present. If this is not done, the program may fail to converge. (It is not necessary to add Ar in process simulation calculations since a gas phase is then almost always present.)
The Gibbs energies of hypothetical pure liquid Fe2C, Mg2C and Ca2C required for the slag model were estimated. The calculated phase diagram is not sensitive to this value for Mg2C and Na4C. The value for Ca2C was estimated from experimental measurements of the Ca distribution coefficients between metal and slag.
Oxycarbonitride Systems
The oxycarbonitride phases in the Al-Si-O-C-N system are schematically shown in Figure 1. The phases that can exist in equilibrium with Al2O3 or Al4C3 are of particular importance for this database. See the calculated diagrams for the Si-Al-O-N system for additional phases not shown in Figure 1.
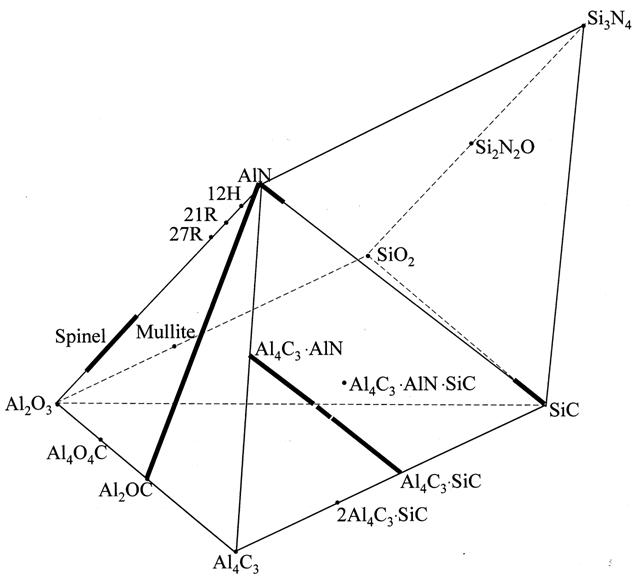
Figure 1. Solid phases in the Al-Si / O-C-N system
The most important parameters of the model for the “Slag” phase are the thermodynamic properties of pure liquid nitrides. The properties of pure solid and liquid AlN and Si3N4 were taken from thermodynamic optimizations. Gibbs energies of liquid Ca3N2, Mg3N2, Fe3N2, and Na3N were estimated from the properties of the corresponding solid compounds (Ca3N2 and Mg3N2) or from the properties of similar solid compounds (Fe3N2 and Na3N) by estimating reasonable temperatures and enthalpies of melting.
Since nitrogen is considered to be a minor element in the FTOxCN database, binary interactions in the liquid solutions ANx-BNy (A, B = Al, Mg, Ca, Fe, Si, Na) were all set equal to zero.
All A2xO‑A3xN and A3xN‑A4xC binary interactions were similarly set to zero except for a few cases when experimental data allowed fixing these parameters.
The Gibbs energies of the exchange reactions
2AlN + 3M2xO = 2M3xN + Al2O3
are very positive for M=Ca, Mg, Fe, Na, and negative when M=Si. Hence, Si will have a slight stabilizing effect on nitrogen in the slags, whereas Ca, Mg, Fe and Na will have negligible effect on the solubility of N in the slags.
The Al2O3–AlN System
Three spinel-related phases close to Al2O3 have been reported. The data on thermodynamic properties and phase equilibria among these phases are too limited to warrant accurate thermodynamic modeling of these phases. Therefore, one spinel phase (AlON) was introduced which covers the range of stoichiometry of all spinel-related phases. Note that the AlON spinel phase expands close to gamma-alumina which is its end‑member.
Three phases with the AlN polytype structure close to the AlN side of the phase diagram are designated as 12H, 21R and 27R in Figure 1. These phases decompose into AlN and spinel below 1891oC. Since the present database is mainly concerned with the phases that can be in equilibrium with alumina, the phases 12H, 21R and 27R are not included. Therefore, the calculated Al2O3–AlN phase diagram is only valid at > 60 mol% Al2O3. The liquidus in the AlN-rich part of the system would be slightly higher if these phases were considered.
The Al4C3–AlN System
This system was optimized taking into account all available experimental data.
The Al2O3–Al4C3–AlN System
The Al2OC-AlN solid solution with the wurtzite-type structure was reported to decompose at low temperatures into phases with the same structure. This could indicate a miscibility gap or formation of an ordered phase. In the absence of reliable experimental information on the nature of the decomposition and on the low-temperature phase equilibria, this decomposition was neglected.
Three quaternary compounds, Al28O21N6C6, Al25O18N9C3, and Al20O10N12C, were reported in one study of this system. Since these findings still have to be confirmed by other studies, and since there is no information on the thermodynamic properties or phase equilibria of these compounds, it was decided not to include these phases in the database.
The Si-Al / C-N System
Al4C3·AlN and Al4C3·SiC form a continuous solid solution (phase name Al4C3(AlN-SiC)) with a primitive hexagonal structure which is interrupted in the central composition region by the formation of the 2Al4C3·AlN·SiC stoichiometric phase. There is one more ternary compound, Al4C3·AlN·SiC.
The Si-Al / O-N System
This system encompasses a major family of oxynitride ceramics, the ‘sialons’, the acronym given to phases in the Si-Al-O-N and related systems that are composed of (Si,Al)(O,N)4 tetrahedra in the same way as the mineral silicates are composed of SiO4 tetrahedra. Each phase in the system extends in a direction where Si+N is gradually replaced by Al+O, the homogeneity ranges perpendicular to this direction being quite small.
The Si-Al-O-N system was completely reoptimized.
For calculated isothermal sections from 1600 to 1900oC, click on “phase diagrams” in this menu. It should be noted that there are six tetrahedral phases in the AlN-rich corner of the phase diagram, the so-called AlN-polytypes. No thermodynamic data are available to reliably model these phases. Only one phase which is closest to Al2O3, the so-called 8H phase, has been included in the present database as a stoichiometric compound Si5Al31O22N23. At higher temperatures, the AlN-polytypes are isolated from alumina by the spinel – 8H, spinel – b’sialon and spinel – slag equilibria. Another ternary phase, which is usually denoted X-phase in sialons, remains in equilibrium with alumina until it melts at about 1727oC. This phase was modeled as a stoichiometric compound Si12Al18O39N8.
The Ca-Al-O-N and Ca-Si-Al-O-N Systems
Experimental information on phase equilibria in these systems is very limited and contradictory. One reciprocal parameter for the liquid slag in the Ca-Al-O-N system was added to reproduce the reported melting temperature of a sample with the Si3N4(CaO)9(AlN)10 composition. For calculated isothermal sections of the Ca-Al-O-N system at 1700oC, click on “phase diagrams” in this menu.
A quaternary stoichiometric compound Ca3Si4Al8O17N4 that can be in equilibrium with alumina was reported at subsolidus temperatures in the Ca-Si-Al-O-N system. The thermodynamic properties of this compound were optimized to reproduce the subsolidus phase relations.
The Mg-Si-O-N and Mg-Ca-Si-O-N Systems
Addition of magnesium to oxynitride systems results in formation of liquid slag at lower temperatures. One parameter of the liquid slag model was introduced to fit a series of melting experiments in the Mg-Si-O-N and Mg-Ca-Si-O-N systems.
Sulfide Systems
Almost no experimental data are available on phase equilibria and thermodynamic properties of phases in the pseudobinary sulfide systems formed by CaS, MgS, FeS, Na2S, SiS2 and Al2S3. Only for the CaS-FeS system some information on the phase diagram has been reported. The thermodynamic properties of the liquid phase and solid compounds were estimated from the properties of the corresponding oxide systems and from the phase diagrams of similar sulfide systems. For the resulted estimated phase diagrams, click on “phase diagrams” in this menu.
Oxysulfide Systems
Almost no experimental data are available on phase equilibria and thermodynamic properties of phases in the oxysulfide systems formed by sulfide (CaS, MgS, FeS, Na2S, SiS2 and Al2S3) and oxide (CaO, MgO, FeO, Na2O, SiO2 and Al2O3) components. Only for the FeO-FeS and FeO-CaS pseudobinary systems some information on the phase diagram has been reported. It was assumed that the liquid phase in the pseudobinary systems MOx-MSx behaves thermodynamically like an ideal Temkin solution (ideal mixing of S2- and O2- on anionic pseudo-sites and metal cations M completely filling the cationic pseudo-sites). For the resulting estimated phase diagrams, click on “phase diagrams” in this menu.
The solubility of sulfur in Al2O3-CaO, SiO2-CaO, SiO2-MgO and SiO2-Na2O slags has been reported. The properties of the slag phase were optimized to reproduce these experimental data.