The Spencer Group SpMCBN Database
for
Refractory Alloy-Carbide, -Nitride, -Boride and -Silicide Systems
General
The Spencer Group Non-Oxide Refractories Database, SpMCBN, contains assessed thermodynamic parameters for binary and ternary alloys of high-temperature materials. These materials are frequently based on transition metal alloys and on their carbides, nitrides, borides, and silicides.
The alloys comprise Me1-Me2-C, Me1-Me2-N, Me1-Me2-B, Me1-Me2-Si, Me-C-N,
Me-C-B, Me-C-Si, Me-N-B, Me-N-Si, Me-B-Si and Me1-Me2-Me3 systems.
The elements included in the Database are:
B, C, N, Si with
Al, Ca, Co, Cr, Fe, Hf, Mg, Mn, Mo, Nb, Ni, Re, Sc, Ta, Tc, Ti, V, W, Y, Zr
The SpMCBN Database version for FactSage 8.3 now includes 2 new binary and 24 new ternary systems. These relate in all but two cases to systems with boron, namely
C-N and C-Y
B-Al-Mn,, B-Al-Y, B-C-Ni, B-C-Y, B-Co-Fe, B-Co-Mn, B-Co-Ni, B-Co-Sc, B-Co-Ti, B-Cr-Mn, B-Cr-Re, B-Fe-Mn, B-Fe-Mo, B-Fe-Ni, B-Fe-Re, B-Fe-Y, B-Mo-Re,
B-Nb-Re, B-Ni-Y, B-Re-W, B-Sc-V, C-Co-Y, C-Fe-Y, C-Ni-Y
In its updated 2023 Version, calculations of thermodynamic properties and phase diagrams can be carried out for ca. 272 binary, and 585 ternary systems, for the individual temperatures or temperature ranges covered by the available experimental information.
In the Database version for FactSage 8.2, modelling of approximately 60 “end-members” associated with several different phases was completed to allow calculations for 4- or higher-component systems. This modelling has continued for boride phases in the present updated database, but such calculations should be viewed as interpolations of somewhat lower accuracy than that associated with the fully assessed lower-order systems. In addition, a small adjustment to the assessed data for the liquid phase of the Nb-C system now avoids calculation of an inverse miscibility gap at very high temperatures in the liquid phase.
The further growth in the number of assessed systems has necessitated the introduction of new elements into existing phases, as well as the self-compatible combination of phases occurring in different binary and ternary systems, but for which the thermodynamic modeling used by different authors is not compatible and often represents differing ranges of stoichiometry.
It is not a realistic proposition to carry out the massive and time-consuming amount of work required to produce self-compatible re-assessments of those systems for which there is incompatibility in the published modeling. For this reason, development of the present database has been achieved, in some cases, by simplification of certain phases, such as the MU and LAVES phases, which often display limited ranges of stoichiometry, to line compounds. This allows calculations for higher-order systems which are still of satisfactory reliability with respect both to thermodynamic properties and phase equilibria.
The simplified modeling used for certain frequently occurring phases observed in refractory alloy systems is exemplified here for the MU phase.
The MU-Phase description in the SpMCBN Database is based on a 3-sublattice model with site occupation 7:2:4. The element occupation of the lattices is
7: Co, Cr, Fe, Mn, Mo, Nb, Ni, Ta, V
2: Mn, Mo, Nb, Ta, Ti, V, W, Zr
4: Co, Cr, Fe, Mn, Mo, Nb, Ni, Ta, Ti, V, W, Zr
Because the MU-Phase has the general stoichiometry of M7X6 (where M and X are the two elements involved) the model does not allow representation of those systems in which Co, Fe, and Ni correspond to X6 in the formula (e.g. Ta7Ni6, Nb7Ni6, Nb7Co6, etc.), because Co, Fe and Ni are not present on the sublattice with occupation 2.
For this reason, the M7C6 phase has been created with stoichiometry M7X6 or X7M6, as a simplification allowing the MU-Phase to be treated as a line compound as and where necessary.
This simplified stoichiometric format, used for phases displaying small ranges of composition allows mixing descriptions in higher-order systems not only for the MU-Phase, but also for other non-stoichiometric phases which appear frequently in refractory metal alloys. Examples of the effect of some simplifications on calculated binary phase equilibria are illustrated in the following diagrams.
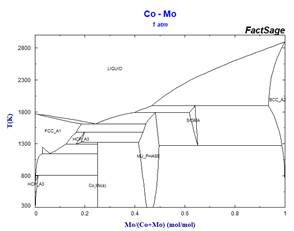
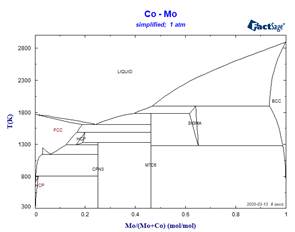
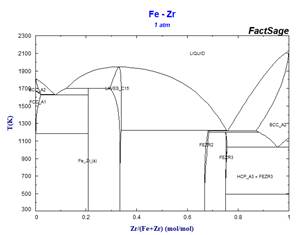
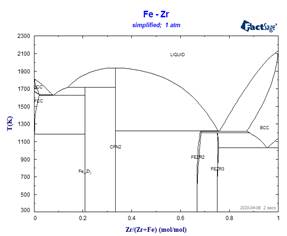
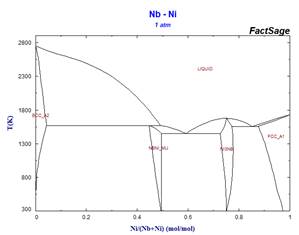
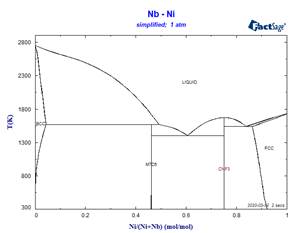
Because these simplifications have relevance for many ternary systems, it has been necessary to rename several phases. This has resulted in revised phase diagram figures in the FactSage Documentation.
Calculations for binary systems are generally not affected by this issue, but when calculating ternary (and higher-order) phase equilibria, it is recommended that the user retain the pre-selected phases used in producing the calculated phase diagrams for the SpMCBN Database shown in the Documentation. All phases relevant for a system of interest can be viewed by selecting ‘File’ in the top left-hand corner of the components page of the Phase Diagram module, then ‘Directories’ and then ‘SpMCBN phase diagrams.’ This will generate a list of all phase diagrams in the SpMCBN Database and choice of a particular system(s) from the list will reveal which compound and solution phase selections were made from the available choices to produce the illustrated phase diagram(s).
The following stoichiometries and the corresponding phase names represent simplifications used frequently in ternary phase diagram calculations.
Phase Stoichiometry Phase Name(s)
A7B2 C7NT
A3B CFN3
A2B CFN2
A7B6 M7C6
AB CFNT
AB2 CNT2
A further development of the SpMCBN Database in FactSage 8.1 was represented by the introduction of volumetric and thermal conductivity parameters for 184 pure solid phases. Volumetric properties (density at 298.15K, volumetric thermal expansivity, compressibility and derivative of the Bulk Modulus) and thermal conductivity parameters (based on the Debye Temperature, Gruneisen Parameter, and atoms per crystallographic cell) are now available for the following carbides, nitrides and borides:
Carbides
|
AlTi2C |
AlTi3C |
Co3AlC |
Cr2AlC |
Cr23C6 |
Cr7C3 |
Cr3C2 |
Fe3AlC |
Hf3Al3C5 |
Hf5Al3C |
|
Hf2Al3C4 |
Mn5C2 |
Mn7C3 |
Mn23C6 |
MoC |
Mo3Al2C |
Mo3Ni3C |
Mo6Ni6C |
Nb2AlC |
Nb4Co2C |
|
Nb4Ni2C |
Sc3C4 |
Sc4C3 |
Ta2AlC |
Ta5Al3C |
Ta4Co2C |
V2AlC |
WC |
Zr3Al3C5 |
Zr5Al3C |
Nitrides
|
h-AlN |
h-BN |
Co4N |
Co2V4N |
Hf3AlN |
Hf5Al3N |
Hf2N |
Hf3N2 |
Hf4N3 |
Mn4N |
|
Mn6N4 |
MnSiN2 |
Nb3Al2N |
Nb4Co2N |
Nb3Fe3N |
Nb4Ni2N |
Ni2V4N |
ScN |
Si3N4 |
TaMoN |
|
TaN_S1 |
Ta4Ni2N |
Ti2AlN |
Ti3AlN |
Ti2N |
WN_S1 |
YN |
Zr3AlN |
Zr5Al3N |
|
Borides
|
AlB2 |
CaB6 |
CoB |
Co2B |
Co3B |
CrB |
CrB2 |
Cr3B4 |
Cr2B |
CrB4 |
|
FeB |
Fe2B |
FeB2 |
HfB2 |
MgB2 |
MgB4 |
MgB7 |
MnB |
MnB2 |
MnB4 |
|
Mn2B |
Mn3B4 |
MoB |
MoB2 |
MoB4 |
Mo2B |
Mo3B2 |
NbB |
NbB2 |
Nb3B2 |
|
Nb3B4 |
NiB |
Ni2B |
Ni3B |
Ni4B3 |
ReB2 |
Re3B |
Re7B3 |
ScB2 |
ScB12 |
|
TaB |
TaB2 |
Ta2B |
Ta3B2 |
Ta3B4 |
TcB2 |
Tc3B |
Tc7B3 |
TiB |
TiB2 |
|
Ti2B |
Ti3B4 |
VB |
VB2 |
V2B3 |
V3B2 |
V3B4 |
W2B |
WB4 |
YB2 |
|
YB4 |
YB6 |
YB12 |
ZrB |
ZrB2 |
ZrB12 |
|
|
|
|
|
AlCr2B2 |
AlCr3B4 |
AlFe2B2 |
Co21Cr2B6 |
Co21Hf2B6 |
Co20V3B6 |
CoVB3 |
Cr5B3 |
CrSc2B6 |
HfCo3B2 |
|
Hf9Mo4B |
Hf2Ni21B6 |
MoAlB |
MoCoB |
Mo2CoB2 |
Mo2Co21B6 |
Mo2NiB2 |
Mo5SiB2 |
MoTiB2 |
MoYB4 |
|
Nb2Co21B6 |
Nb3Co4B7 |
Nb3Co5B2 |
NbFeB |
Ni20All3B6 |
NiCr3B6 |
Ni3Cr2B6 |
NiScB4 |
Ni21Sc2B6 |
Ni6Si2B |
|
Re3Al2B |
ReCoB |
Re5Co2B4 |
ReTi2B2 |
ReYB4 |
ReY2B6 |
ReY3B7 |
Re4YB4 |
Ta2Co21B6 |
Ta3Co5B2 |
|
Ta2Ni21B6 |
WCoB |
W2CoB2 |
W2Co21B6 |
W2FeB2 |
W3Hf9B2 |
W2NiB2 |
WY3B7 |
YCoB4 |
YCo2B2 |
|
YCo3B2 |
YCo4B |
YCo12B6 |
YCrB4 |
Y5Si2B8 |
Zr2Co21B6 |
Zr2Ni21B6 |
|
|
|
The database is based on, and is compatible with, assessed data for relevant binary, and some ternary systems from the SGTE2014 Solution Database, but the SpMCBN Database incorporates thermodynamic parameters for very many previously un-assessed systems.
The general procedure used in obtaining assessed parameters for the solution and compound phases of the large number of previously un-assessed ternary systems in the SpMCBN Database has been to combine the phase boundary information contained in the ASM Handbook of Ternary Alloy Phase Diagrams, 1995, and in the more recent ASM Alloy Phase Diagram Database, 2016, with available assessed thermodynamic data for the appropriate binary sub-systems. This has allowed a completely compatible set of values to be derived to describe binary and ternary thermodynamic properties and phase equilibria for a particular system. Because the ASM compilations often present, for a given binary or ternary system, several published phase diagrams which differ from each other, those authors of the diagram to which greatest weight has been given in the present assessment work, are listed in the references accompanying the database.
There is a great scarcity of published experimental thermodynamic values for the phases of ternary systems, especially for refractory alloys. For this reason, the simplification of additivity of element entropy and heat capacity data has been used frequently as the basis for obtaining a complete set of thermodynamic values for ternary compounds. Enthalpies of formation and standard entropies of the compounds have then been derived to give consistency with the published phase equilibria.
While no data for oxide systems have been included in the database, several of the elements
listed above are important in refractory oxide materials. Reactions of the alloy, carbide, nitride, boride and silicide systems with such refractory oxides and with oxygen-containing gas atmospheres can be calculated using FactSage by selecting the SpMCBN database together with appropriate combinations of the FToxid, FACTPS and SGPS databases for the materials in question.
The significant experimental difficulties associated with the determination of phase equilibria and thermodynamic properties of materials at high temperatures result in a generally lower accuracy of the assessed thermodynamic parameters for refractory systems. Many of the published experimental ternary phase diagrams originate from work carried out some 30 or more years ago, and in some cases now show incompatibility with accepted binary phase equilibria. However, the data in the SpMCBN database are believed to allow generally reliable calculation of thermodynamic properties and phase equilibria for the temperatures and temperature ranges covered by the phase diagrams accompanying the documentation of this database.
The reliability of the assessment for an individual binary or ternary system is summarized under “assessments” in the documentation for the SpMCBN Database. All the assessed systems are presented with colour-coding for the quality of the assessment, in tables headed
SpMCBN Binary Assessments
Ternary Systems with Boron
Ternary Systems with Carbon
Ternary Systems with Nitrogen
Ternary Systems with Silicon
Ternary Alloy Systems without B, C, N or Si.
The term “refractory” applied to materials with applications in high-temperature structures or equipment is somewhat indefinite, but generally implies a use above about 1000°C (1273K).
The major applications of the SpMCBN Database relate to the ever-expanding field of non-oxide refractories based on carbides, nitrides, borides and silicides. These are finding wide use, in combination with appropriate elements, to produce hard, high melting temperature materials. Some examples of use are in furnace construction, high-temperature coatings, cutting tools, abrasives, aircraft brake linings, rockets, jets, turbines, and nuclear power plants.
Specific information on the phases present in each alloy system and references to the individual unary, binary and ternary systems in the database can be obtained by consulting the documentation for the SpMCBN Database.
N.B. The SpMCBN Database includes many of the elements which are important in steels. It is emphasized, however, that for calculations related to the production and heat-treatment of steels, the FSstel Database should be selected. The assessed data contained in FSstel are based on the much greater amount of accurate experimental data available specifically for steel compositions, than are available for refractory Fe-containing alloys.
Further information on the development and application of the SpMCBN Database can be found in the following two references:
P.J. Spencer, Development of a thermodynamic database for refractory boride, carbide, nitride and silicide systems, Proceedings Materials Science and Technology 2017 (MS&T17), 1230-1238.
S. Saxena, P. Spencer, V. Drozd, An alternative, environmentally-friendly production process for refractory metal carbides and syngas using methane reduction of the oxide ores, Monatshefte für Chemie in memoriam Heinz Gamsjäger, 149 (2018) 411-422.